Search

Search










Potassium benzylpenicillin has a good antibacterial effect on streptococcus species such as hemolytic streptococcus, streptococcus pneumoniae and staphylococcus, which does not produce penicillinase.
Potassium benzylpenicillin is indicated for various infections caused by sensitive bacteria, such as abscesses, bacteremia, pneumonia and endocarditis. Penicillin has good antibacterial effect on streptococcus such as hemolytic streptococcus, Streptococcus pneumoniae and Staphylococcus that does not produce penicillinase. It has a moderate antibacterial effect on enterococci.
| Items | Specification | Result |
Assay | 96.0%-102.0% | 101.6% |
Appearance | White or almost white crystalline powder | Complies |
pH | 5.5-7.5 | 6.6 |
Loss on drying | ≤1.0% | 0.21% |
| Product parameters | |
| Cas number: | 113-98-4 |
| Appearance: | White or almost white crystalline powder |
| Purity: | 96.0%-102.0% |
| Package details: | 10BOU/bag,2bag/drum |
| Brand: | Fortunachem |
Penicillin G Potassium Salt is the potassium salt form of Benzylpenicillin (Penicillin G), the first and most widely used natural penicillin antibiotic. Chemically, it is derived from the fermentation of the fungus Penicillium chrysogenum.
Chemical Structure & Properties:
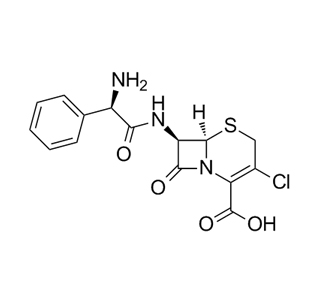
Chemical Name: (2S,5R,6R)-3,3-Dimethyl-7-oxo-6-[(phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, potassium salt.
Molecular Formula: C₁₆H₁₇KN₂O₄S
Key Features: It consists of a β-lactam ring fused to a thiazolidine ring (the core penicillin nucleus) and a side chain (phenylacetyl group). The potassium cation provides water solubility.
Solubility: Highly soluble in water, but unstable in aqueous solution (hydrolyzes easily). It is poorly soluble in organic solvents like ethanol or chloroform.
In a chemical context, Penicillin G potassium salt is primarily used as a starting material, reference standard, or substrate in various chemical and biochemical processes. Its medical use as an antibiotic is distinct from these chemical applications.
Substrate for β-Lactamases: It is a classic, natural substrate used to study the activity, kinetics, and inhibition of β-lactamase enzymes (the bacterial enzymes that confer resistance by hydrolyzing the β-lactam ring). Researchers use it to test the efficacy of β-lactamase inhibitors like clavulanic acid.
Studies on Penicillin-Binding Proteins (PBPs): Used to investigate the interaction between β-lactam antibiotics and their target enzymes (PBPs) in bacterial cell wall synthesis.
Precursor for Semisynthetic Penicillins: It is the fundamental starting material for the production of other penicillins.
The side chain can be enzymatically cleaved (e.g., using penicillin acylase) to yield 6-aminopenicillanic acid (6-APA).
6-APA is a crucial scaffold to which different side chains can be chemically attached to synthesize a wide array of semisynthetic penicillins with improved properties (e.g., ampicillin, amoxicillin).
Model Compound: Used in organic chemistry to study the reactivity of the strained β-lactam ring, including its hydrolysis and reactions with nucleophiles.
Reference Standard: Highly purified Penicillin G potassium is used as a pharmacopoeial reference standard (USP, EP) to:
Calibrate instruments (HPLC, UV-Vis).
Identify and quantify Penicillin G in samples (e.g., fermentation broths, pharmaceutical formulations, milk, or animal tissues) for quality control and regulatory testing.
Develop and validate analytical methods.
Model Fermentation Product: As a classic product of microbial fermentation, it is studied to optimize fermentation conditions, precursor feeding (e.g., phenylacetic acid), and metabolic pathways in industrial microbiology.
Educational Tool: Used in teaching labs to demonstrate principles of:
Antibiotic susceptibility testing (disk diffusion assays).
Enzyme kinetics (β-lactamase assays).
Basic techniques in pharmaceutical chemistry and microbiology.
Instability: The β-lactam ring is highly susceptible to hydrolysis in both acidic and basic conditions, as well as by heat and heavy metals. Solutions must be prepared fresh and used immediately.
Hygroscopic: The potassium salt form absorbs moisture from air, requiring storage in a dry, sealed container.
Allergenicity: Even in a lab setting, it is a potent allergen. Proper personal protective equipment (gloves, mask, eyewear) is essential to prevent sensitization.
While clinically it is an antibiotic for treating gram-positive infections, chemically, Penicillin G potassium salt serves as:
A key biochemical substrate for resistance enzyme studies.
The essential chemical precursor for manufacturing other penicillins.
A critical analytical standard for identification and quantification.
A fundamental tool in fermentation science and chemical education.
Its value in chemistry lies in its unique, reactive β-lactam structure and its role as the progenitor molecule for a vast class of semisynthetic antibiotics.


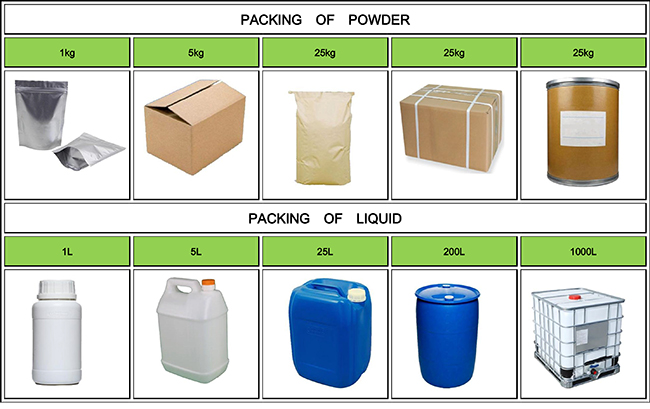

High quality & competitive price
Quality control
Fast feedback
Prompt shipment

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية