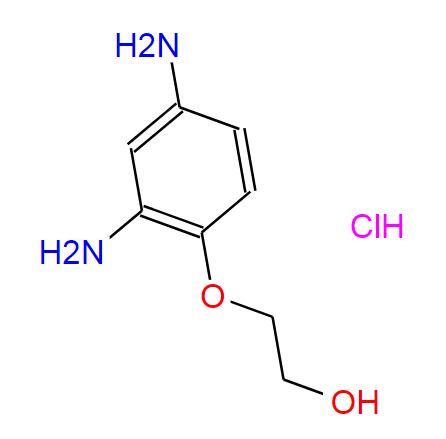
Search

Search











Items | Specifications | Results |
Appearance | White crystalline powder | |
Assay | ≥99.0-100.5% | 99.65% |
Melting point | 41~43℃ | 42℃ |
Chloride | ≤100PPM | 70PPM |
Sulfate | ≤100PPM | 70PPM |
Heavy metals | ≤15PPM | 10PPM |
Ignition residue | ≤0.10% | 0.10% |
Loss of drying | ≤1% | 0.45% |
Conclusion | The product conforms to the above specifications. | |
Phenyl salicylate, commonly known by its historical trade name Salol, is an organic compound with the chemical formula C₁₃H₁₀O₃. It is the phenyl ester of salicylic acid. Structurally, it consists of a phenyl ring (benzene ring) attached via an ester linkage to a salicylate group.
Basic Properties:
Appearance: White, crystalline powder or colorless crystals.
Odor: Faint, characteristic, wintergreen-like odor.
Melting Point: ~43°C (109°F). It's known for its low melting point.
Key Characteristic: It is hydrolyzed in the small intestine, breaking down into salicylic acid and phenol.
This was its original and most famous use, pioneered by Marceli Nencki in the 1880s.
Enteric Coating/Prodrug: Salol's core medicinal property was that it remained intact in the acidic environment of the stomach but hydrolyzed in the alkaline environment of the small intestine. This served two purposes:
To protect the stomach from the direct irritant effect of salicylic acid.
To act as a prodrug for the systemic release of salicylic acid (an analgesic and antipyretic) and phenol (a mild antiseptic).
Why Obsolete? Safer, more effective drugs like acetylsalicylic acid (aspirin) and modern enteric coatings replaced it. The release of phenol, which is toxic in higher doses, was a significant downside.
These are its main applications today:
Polymer Industry:
Ultraviolet Light Absorber & Light Stabilizer: It is added to plastics (like PVC, polyethylene, and acrylics) to absorb harmful UV radiation, preventing yellowing, degradation, and loss of mechanical strength.
Dye Carrier: Used in the dyeing of synthetic fibers.
Odor Agent & Perfumery:
Due to its mild, pleasant odor, it is used as a fixative in perfumes and soaps to slow the evaporation of more volatile fragrance components.
Sometimes labeled as a "fragrance ingredient."
Laboratory Reagent:
Used in chemical synthesis and as a standard in various laboratory experiments, particularly those demonstrating ester hydrolysis or pH-dependent reactions.
Medical Demonstrations:
Its pH-dependent hydrolysis is still used in educational settings (e.g., the "Salol Test") to illustrate how enteric coatings work or to roughly determine gastrointestinal transit time.
Sunscreen Preparations: Rarely, due to its UV-absorbing properties, though much more effective modern filters are standard.
Antiseptic in Topical Ointments: Very limited use, largely historical.
Synthesis: It is typically produced by the esterification reaction of salicylic acid with phenol.
Safety: Generally considered low toxicity for external use in industrial applications. However, ingestion is hazardous due to the release of phenol. It can cause nausea, vomiting, tinnitus, and central nervous system effects. It is not meant for modern self-medication.
Regulatory Status: Approved for use in cosmetics and industrial applications within specified limits, but not as an active drug ingredient in modern pharmacopeias.
| Category | Primary Use | Mechanism/Reason |
|---|---|---|
| Historical | Oral Analgesic/Antipyretic | Enteric prodrug releasing salicylic acid & phenol. |
| Industrial | UV Stabilizer for Plastics | Absorbs UV light, preventing polymer degradation. |
| Industrial | Fragrance Fixative & Odor Agent | Provides a mild, persistent wintergreen-like scent. |
| Industrial | Dye Carrier | Aids in dyeing synthetic fibers. |
| Educational | Laboratory Demonstration | Shows ester hydrolysis and enteric coating principles. |
In essence, phenyl salicylate has transitioned from a pioneering 19th-century drug to a modern industrial chemical, primarily valued for its ability to protect plastics from sunlight and for its mild fragrance.




Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية