Search

Search









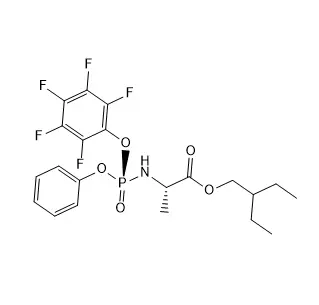
2,3,4,6-Tetra-O-acetyl-beta-D-galactopyranosyl azide is a chemically modified galactose derivative. Its six-membered sugar ring (β-D-galactopyranose) has acetyl groups (-OAc) protecting hydroxyls at positions 2, 3, 4, and 6, enhancing stability. The anomeric carbon (C1) bears an azide (-N₃) group in the β-configuration. Synthesized via SN2 substitution of a galactosyl halide with sodium azide, it serves as a glycosyl donor in carbohydrate synthesis. The azide enables "click chemistry" (e.g., CuAAC reactions) for bioconjugation or drug delivery. Acetyl groups are later removed to expose reactive hydroxyls. Handle carefully—organic azides may decompose under heat or shock. Widely used in glycobiology and pharmaceutical research.
2,3,4,6-Tetra-O-acetyl-beta-D-galactopyranosyl azide is a chemically modified derivative of D-galactose, designed for use in synthetic chemistry, particularly in glycosylation and conjugation reactions. Here's a detailed breakdown:
Galactopyranosyl Backbone:
The core structure is D-galactose in its pyranose form (a six-membered ring consisting of five carbons and one oxygen).
The "beta" configuration indicates the azide group (-N₃) at the anomeric carbon (C1) is positioned trans (opposite) to the CH₂OH group at C5 in the Haworth projection.
Acetyl Protecting Groups:
Hydroxyl groups at positions 2, 3, 4, and 6 are acetylated (-OAc), rendering them inert. This protection enhances stability and directs reactivity to the anomeric position.
Azide Functional Group:
The anomeric carbon (C1) bears an azide group, making this compound a glycosyl azide. This group is highly reactive in click chemistry (e.g., CuAAC) or reducible to an amine for further functionalization.
Preparation: Typically synthesized from peracetylated galactosyl halides (e.g., galactosyl bromide) via nucleophilic substitution (SN2) with sodium azide (NaN₃).
The β-configuration arises from backside attack by the azide ion on the α-configured halide, leading to inversion of stereochemistry.
Acetyl groups act as electron-withdrawing substituents, suppressing neighboring group participation and favoring SN2 mechanisms.
Click Chemistry:
The azide group enables copper-catalyzed azide-alkyne cycloaddition (CuAAC) for bioorthogonal labeling or glycoconjugate synthesis.
Glycosylation Reactions:
Serves as a glycosyl donor in oligosaccharide synthesis. The azide can later be reduced to an amine (-NH₂) for additional coupling steps.
Protecting Group Strategy:
Acetyl groups are easily removed (e.g., via Zemplén deacetylation with NaOMe/MeOH) to regenerate free hydroxyls post-conjugation.
Azide Stability: While stabilized by acetylation, azides can decompose exothermally under heat or shock. Proper handling and storage are essential.
This compound is a versatile intermediate in organic and carbohydrate chemistry, combining the reactivity of an azide with the protective stability of acetyl groups. Its primary utility lies in constructing complex glycostructures or biomolecular conjugates through efficient, selective reactions.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية