
Search

Search

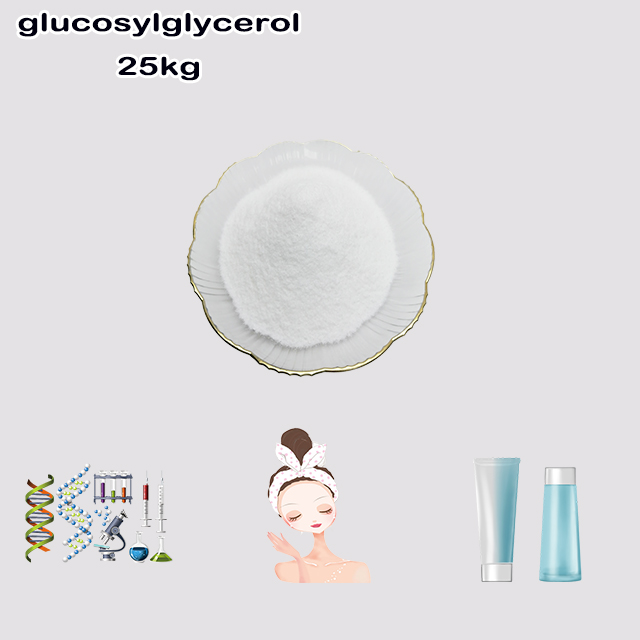









Here's a concise explanation of glucosylglycerol:
Glucosylglycerol (GG) is a natural glycoside and compatible solute (osmolyte). Chemically, it consists of one molecule of glucose linked via an α-glycosidic bond to the primary hydroxyl group of glycerol (molecular formula: C₉H₁₈O₈).
Its key features are:
Highly water-soluble and neutral (no charge).
Primary function: Accumulates in cells (especially cyanobacteria and some microbes) to protect against environmental stresses like high salt (osmotic stress), drought, and heat. It stabilizes proteins and membranes without disrupting function.
Applications: Explored for stabilizing enzymes/proteins in biotechnology, cosmetics (skin moisturizing), and potentially as a stress protectant in agriculture.
Glucosylglycerol (GG) is a naturally occurring compatible solute (osmolyte) with the chemical structure of a glycoside. Here's a detailed breakdown of its chemistry:
Chemical Classification:
Disaccharide Analog: While not a true disaccharide (which would be two monosaccharides), it consists of one glucose molecule and one glycerol molecule linked together.
Glycoside: Specifically, it's a glucosylglycerol because glucose is the glycone (sugar) part and glycerol is the aglycone (non-sugar) part.
Chemical Structure:
The anomeric carbon (C1) of glucose (in its α-configuration) is linked.
The linkage is to the primary hydroxyl group (specifically, the sn-1 or sn-3 position) of glycerol.
This forms an α-1,2-glycosidic bond (if linking to the terminal carbon of glycerol) or more generally just an O-α-D-glucopyranosyl-(1→X)-glycerol (where X indicates the glycerol carbon involved).
D-Glucose: A hexose (6-carbon) monosaccharide (pyranose form).
Glycerol: A 3-carbon polyol (sugar alcohol).
Core Components:
Linkage: The glucose and glycerol are connected via a glycosidic bond. The most common and biologically relevant form is α-Glucosylglycerol, where:
Molecular Formula: C₉H₁₈O₈
Systematic Name (IUPAC): The preferred IUPAC name for the common α-isomer is:
(2R,3R,4S,5S,6R)-2-(Hydroxymethyl)-6-[(2R)-2,3-dihydroxypropoxy]oxane-3,4,5-triol
(This describes the stereochemistry of the glucose ring and the specific chiral center of glycerol involved in the linkage).
Simpler Name: 1-O-α-D-Glucopyranosyl-glycerol or 2-O-α-D-Glucopyranosyl-glycerol (depending on which glycerol carbon is linked; the 1-O form is most common). The term α-Glucosylglycerol is widely used.
CAS Number: 131352-92-8 (commonly cited for the mixture/isomers), but specific stereoisomers have their own numbers.
Key Chemical Properties:
Not interacting strongly with proteins or membranes.
Stabilizing proteins and membranes under stress conditions (e.g., high salt, high temperature, drought).
Acting as an osmolyte to balance osmotic pressure.
Highly Water-Soluble: Due to its multiple hydroxyl (-OH) groups.
Hydrophilic: Strong affinity for water.
Low Molecular Weight: MW = 254.24 g/mol.
Non-Ionic (Neutral Charge): At physiological pH, it carries no net charge. However, it can act as a very weak acid/base due to its hydroxyl groups.
Stable: Relatively chemically stable under physiological conditions.
Compatible Solute: Its defining characteristic. It accumulates intracellularly in certain microorganisms without disrupting cellular functions, even at very high concentrations. It achieves this by:
Reducing Sugar?: The anomeric carbon of glucose is involved in the glycosidic bond. Therefore, glucosylglycerol itself is not a reducing sugar, as it lacks a free anomeric carbon capable of forming an open-chain aldehyde.
Stereochemistry:
The glucose moiety has specific stereochemistry (D-glucose, α-anomeric configuration at the linkage point).
Glycerol itself is achiral, but the carbon atom attached to the glycosidic oxygen becomes chiral upon substitution (if linked to a primary OH). The natural product is usually enantiomerically pure (R configuration at this glycerol carbon in the common 1-O-α-D-Glucopyranosyl-sn-glycerol form).
Natural Occurrence & Significance:
Primarily found in cyanobacteria (blue-green algae), some other bacteria, and a few extremely salt-tolerant (halophilic) eukaryotic microorganisms.
It's their primary osmolyte for surviving high salinity (salt stress) and other environmental stresses like drought or high temperature.
Its chemical structure (neutral, hydrophilic, compatible) is evolutionarily optimized for this protective role.
Distinction from Similar Compounds:
Glucosylglycerate: Has glyceric acid (glycerate) instead of glycerol, carrying a negative charge.
Trehalose: A true disaccharide (glucose + glucose).
Sucrose: A true disaccharide (glucose + fructose).
In Summary: Chemically, glucosylglycerol (C₉H₁₈O₈) is a neutral glycoside formed by linking the anomeric carbon of α-D-glucose to a primary hydroxyl group of glycerol via an O-glycosidic bond. Its key defining chemical properties are high water solubility, hydrophilicity, low molecular weight, neutrality, stability, and its exceptional function as a compatible solute/osmolyte due to its specific structure and stereochemistry.
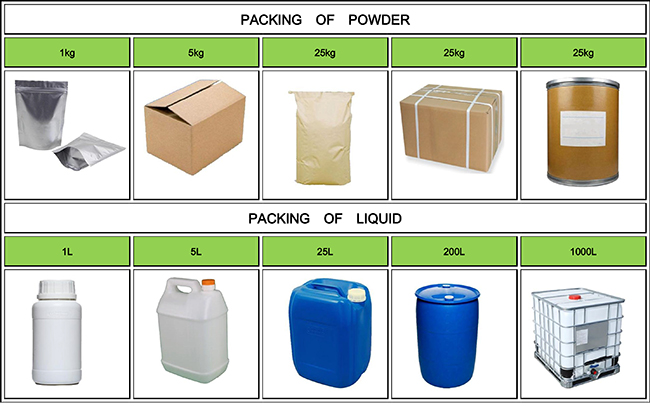



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
_A25主图.jpg)


Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية 


