
Search

Search











1,2-Hexanediol (C₆H₁₄O₂) is a clear, colorless synthetic liquid diol widely used in cosmetics and industrial applications. Key points:
Dual Solubility:
Soluble in water and oils (unlike shorter-chain diols), making it versatile.
Humectant:
Binds moisture in skincare (serums, creams) to prevent dryness.
Preservative Booster:
Enhances efficacy of antimicrobials (e.g., phenoxyethanol) against bacteria/fungi.
Solvent & Carrier:
Dissolves actives (vitamins, pigments) and improves product texture.
Cosmetics: Moisturizers, sunscreens, makeup (replaces glycerin for lighter feel).
Cleansers: Lowers surface tension for better oil removal.
Industrial: Coatings, inks, antifreeze.
Low Irritation: Safe for sensitive skin (OECD-tested).
Biodegradable: Environmentally compliant.
Labeling: Often listed as "1,2-Hexanediol" in INCI.
Why Formulators Use It:
Multifunctional (reduces need for multiple additives).
Extends shelf life without parabens.
Improves spreadability without stickiness.
1,2-Hexanediol (C₆H₁₄O₂) is a saturated vicinal diol (a 6-carbon chain with two adjacent hydroxyl groups). Here are its key chemical characteristics:
IUPAC Name: *Hexane-1,2-diol*
Molecular Formula: C₆H₁₄O₂
Structural Formula:
HO−CH₂−CH(OH)−CH₂−CH₂−CH₂−CH₃
Vicinal diol (OH groups on C1 and C2).
Chiral center at C2 (exists as R/S enantiomers; typically used as racemate).
Physical State: Colorless, viscous liquid (mp: −10°C; bp: 223°C).
Solubility:
Miscible in water (forms H-bonds).
Soluble in organic solvents (ethanol, acetone) and oils (log P: −0.25).
Hydrophilic-Lipophilic Balance (HLB): ~5.3 (moderate surfactant properties).
Hydrogen Bonding: Strong H-bond donor/acceptor → humectancy.
Reactivity:
Dehydration: Forms aldehydes/ketones under acid catalysis.
Esterification: Reacts with acids to yield esters (e.g., in polymer synthesis).
Coordination Chemistry: Binds metal ions (e.g., as a chelator).
Preservative Mechanism: Disrupts microbial membranes via OH group interactions.
Industrial Route:
Hydroxylation of 1-Hexene with peracetic acid:
CH2=CH−CH2CH2CH2CH3+CH3COOOH→HO−CH2−CH(OH)−CH2CH2CH2CHCHX2=CH−CHX2CHX2CHX2CHX3+CHX3COOOH ——>
HO−CHX2−CH(OH)−CHX2CHX2CHX2CHX3
| Role | Chemical Basis |
|---|---|
| Humectant | H-bonding with water → retains moisture |
| Solvent/Carrier | Dual solubility (polar/non-polar) |
| Preservative Aid | Disrupts microbial membrane integrity |
| Viscosity Modifier | H-bonding network → thickens solutions |
IR: Broad O−H stretch (3200–3400 cm⁻¹); C−O stretch (1050–1100 cm⁻¹).
NMR (¹H):
C1−OH: δ 3.5–3.7 ppm (t, 2H)
C2−OH: δ 3.3–3.5 ppm (m, 1H)
C2−H: δ 3.4–3.6 ppm (m, 1H)
Low Toxicity: LD₅₀ (oral, rat) >2000 mg/kg.
Biodegradable: Readily metabolized via β-oxidation.
Non-Irritating: Safe for cosmetic use (OECD 439).
Why Chemists Value It: Combines water solubility (from vicinal OH) with lipophilic compatibility (C4-C6 alkyl chain), enabling versatile formulations. Its dual functionality reduces the need for multiple additives, aligning with "clean chemistry" trends.


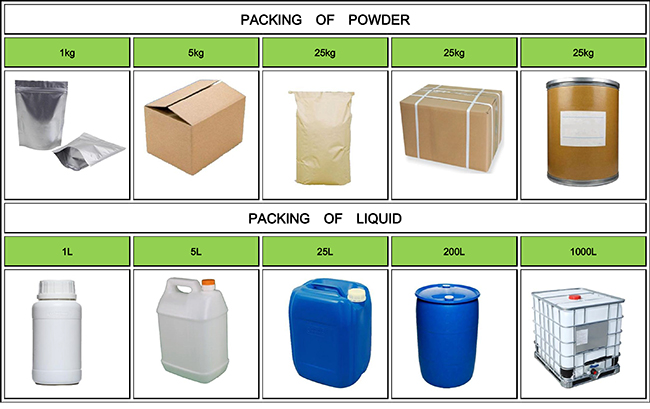

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية 






