Search

Search











Aluminum Stearate is a metallic soap with the general formula Al(C18H35O2)3 or Al(OH)(C18H35O2)2, formed by reacting stearic acid (a saturated fatty acid) with aluminum salts.
Structure: A complex of aluminum ions bound to stearate anions (long-chain carboxylates).
Appearance: White, waxy powder, insoluble in water but soluble in oils and organic solvents.
Function: Acts as a thickening/gelling agent in hydrophobic systems due to its ability to form colloidal networks.
Lubricants: Thickens greases (e.g., lithium-based greases).
Cosmetics/Pharma: Stabilizes emulsions, prevents caking in powders.
Paints/Coatings: Modifies viscosity, prevents pigment settling.
Waterproofing: Used in textiles, concrete, or candles.
Generally low toxicity but may irritate eyes/respiratory tract.
Environmentally benign but persistent in large quantities.
Its amphiphilic nature (hydrophobic tail + polar head) makes it versatile in industrial formulations.
Aluminum Stearate is a metallic soap formed by the reaction of stearic acid (a long-chain fatty acid) with aluminum salts. Here's a structured overview:
Formula: Typically represented as Al(C18H35O2)3Al(C18H35O2)3 , indicating one aluminum ion (Al³⁺) bound to three stearate anions.
Structure: Combines the trivalent aluminum ion with stearate, the conjugate base of stearic acid (C₁₇H₃₅COOH).
Physical Form: White, waxy powder or solid.
Solubility: Insoluble in water but soluble in organic solvents; forms gels in oils and hydrocarbons.
Hydrophobicity: Exhibits water-repellent properties due to the long hydrocarbon chain of stearic acid.
Synthesis: Produced by reacting stearic acid with aluminum hydroxide (Al(OH)3) or aluminum chloride (AlCl3), yielding the aluminum salt and water or HCl as byproducts.
Cosmetics & Pharmaceuticals:
Thickening Agent: Adjusts viscosity in creams and ointments.
Stabilizer: Maintains emulsion consistency (e.g., lotions).
Anti-Caking Agent: Prevents clumping in powders.
Paints & Coatings:
Thixotropic Agent: Prevents pigment settling and improves texture.
Water Repellent: Enhances moisture resistance.
Plastics & Polymers:
Lubricant: Reduces friction during processing.
Release Agent: Aids in mold separation.
Other Uses:
Waterproofing: Used in textiles and construction materials.
Greases: Acts as a thickening agent in lubricating greases.
Regulations: Generally recognized as safe in low concentrations, though aluminum exposure debates (e.g., neurotoxicity concerns) may limit certain uses.
Handling: Non-toxic but may cause irritation in excessive amounts; subject to industry-specific safety guidelines.
Commercial variants may include mixed fatty acid salts (e.g., palmitate) alongside stearate.
Its versatility stems from gel-forming ability, hydrophobicity, and thermal stability.
In summary, Aluminum Stearate is a multifunctional compound widely utilized for its thickening, stabilizing, and hydrophobic properties across diverse industries.

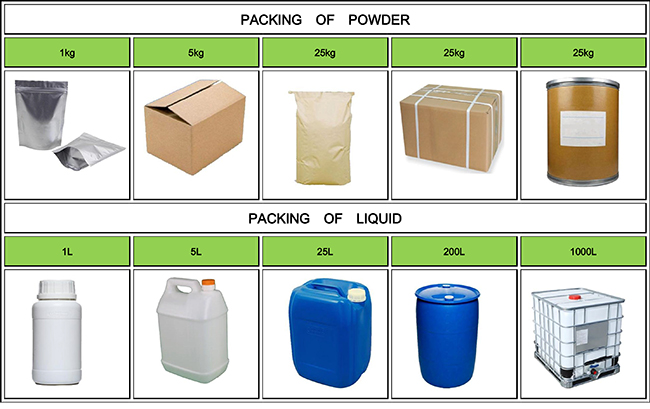


Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية