Search

Search











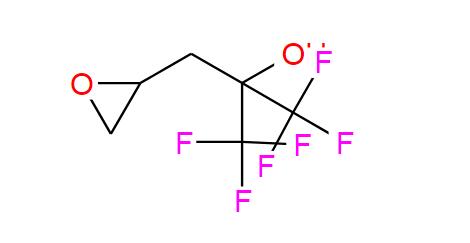
2-Mercaptobenzothiazole (MBT) is an organosulfur compound with the formula C₇H₅NS₂. It consists of a benzothiazole ring (a fused benzene and thiazole ring) with a sulfhydryl (-SH) group at the 2-position. Key features:
Applications: Primarily used as a vulcanization accelerator in rubber production (tires, hoses) to enhance curing. Also acts as a corrosion inhibitor in metalworking fluids and a fungicide/bacteriocide in adhesives and coatings.
Safety: Can cause skin allergies (contact dermatitis) and is toxic to aquatic life. Regulated under REACH in the EU.
Handling: Requires protective gear (gloves, goggles) due to irritant properties.
A versatile but hazardous industrial chemical.
2-Mercaptobenzothiazole (MBT) is an organosulfur compound with the chemical formula C₇H₅NS₂. It belongs to the benzothiazole class, characterized by a fused benzene and thiazole ring system. Here's a detailed breakdown:
Core Structure: A benzothiazole ring, which is a bicyclic structure consisting of a benzene ring fused to a thiazole (a five-membered ring containing sulfur and nitrogen).
Functional Group: A thiol (-SH) group is attached at the 2-position of the benzothiazole ring. This position corresponds to the carbon adjacent to the sulfur atom in the thiazole moiety.
Physical Form: Typically a pale yellow to light brown crystalline powder.
Melting Point: ~180°C.
Odor: Slight sulfurous odor.
Solubility: Insoluble in water but soluble in organic solvents like ethanol and acetone.
Rubber Industry:
Vulcanization Accelerator: MBT is widely used to accelerate the sulfur vulcanization process in rubber production, enhancing elasticity and durability.
Applications: Tires, hoses, belts, and other rubber products.
Corrosion Inhibition:
Acts as a corrosion inhibitor in metalworking fluids and coatings.
Biocides:
Used in antifouling paints and fungicides due to its antimicrobial properties.
Coordination Chemistry:
Serves as a ligand for metal ions, forming complexes in analytical and industrial processes.
Allergen: A known skin sensitizer, causing contact dermatitis in exposed individuals (e.g., rubber industry workers).
Toxicity: Moderate toxicity via ingestion or inhalation; requires proper handling and protective equipment.
Persistence: Can persist in the environment, potentially affecting aquatic ecosystems.
Regulations: Regulated under frameworks like the EU REACH due to its allergenic and environmental risks.
Produced via reactions involving aniline, carbon disulfide, and sulfur under controlled conditions, followed by cyclization to form the benzothiazole ring.
Other accelerators like thiurams or dithiocarbamates may replace MBT in specific applications to mitigate health risks.
MBT is a critical compound in rubber manufacturing with additional roles in corrosion prevention and biocides. While industrially valuable, its use necessitates careful management to address health and environmental concerns.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية