Search

Search











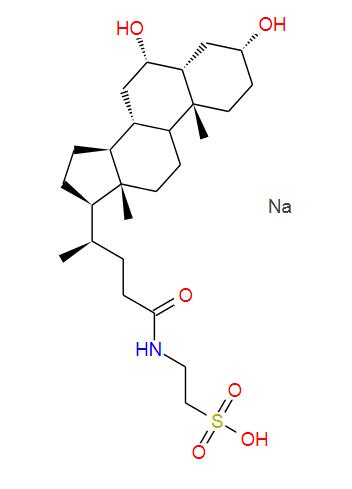
Phlorizin (CAS 60-81-1) is a natural dihydrochalcone glycoside found in apple tree bark and roots. It inhibits sodium-glucose co-transporters (SGLT) in kidneys and intestines, blocking glucose reabsorption and inducing glucosuria. Historically used to study diabetes, it inspired modern SGLT2 inhibitor drugs for type 2 diabetes treatment. It also exhibits antioxidant properties and has been used in traditional medicine for antimicrobial effects. Though non-selective (affects SGLT1/SGLT2), it remains vital in glucose metabolism research. Handle with care; high doses may cause dehydration. Molecular formula: C₂₁H₂₄O₁₀.
Phlorizin (CAS 60-81-1) is a naturally occurring phenolic compound classified as a dihydrochalcone glycoside. Here’s a detailed breakdown:
IUPAC Name: 1-[2-(β-D-Glucopyranosyloxy)-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one
Molecular Formula: C₂₁H₂₄O₁₀
Molecular Weight: 436.41 g/mol
Appearance: White to off-white crystalline powder.
Chemical Structure:
Composed of phloretin (a dihydrochalcone) linked to a β-D-glucose moiety via a glycosidic bond.
Contains multiple hydroxyl (-OH) groups, contributing to its antioxidant properties.
Primarily found in the bark and roots of apple trees (Malus domestica) and other Rosaceae plants.
Acts as a plant defense compound against pathogens and herbivores.
SGLT Inhibition:
Potent inhibitor of sodium-glucose co-transporters (SGLT1 and SGLT2) in the kidneys and intestines.
Blocks glucose reabsorption, leading to glucosuria (excretion of glucose in urine).
Diabetes Research:
Historically used to induce "chemical diabetes" in animal models for studying glucose metabolism.
Mechanism inspired the development of SGLT2 inhibitor drugs (dapagliflozin) for type 2 diabetes.
Research Tool:
Used to study renal glucose handling, insulin resistance, and gut glucose absorption.
Antioxidant:
Scavenges free radicals due to its phenolic structure.
Traditional Medicine:
Extracts containing phlorizin were historically used for antimicrobial and anti-inflammatory purposes.
Toxicity: Generally low toxicity in research settings, but high doses may cause dehydration or electrolyte imbalance (due to glucosuria).
Precautions: Use gloves and eye protection when handling; avoid inhalation or ingestion.
Historical Impact: Phlorizin was critical in early diabetes research, helping elucidate the role of renal glucose transporters.
Modern Relevance: While not used clinically due to non-selectivity (inhibits both SGLT1 and SGLT2), it remains a key reference compound for developing selective SGLT2 inhibitors.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية