Search

Search











Ethyl Salicylate (C₉H₁₀O₃) is a natural ester derived from salicylic acid and ethanol.
Properties: Colorless liquid with a sweet, wintergreen-like aroma.
Key Uses:
Fragrance: In perfumes, soaps, and cosmetics.
Topical Analgesic: Relieves muscle/joint pain (converted to salicylic acid on skin).
Flavoring: FDA-approved for candies/gums (low doses).
Safety: Generally safe in dilution; avoid undiluted skin contact/ingestion (potential irritant).
Natural Sources: Wintergreen, cloves, strawberries.
Ideal for:
Aromatherapy blends • Pain-relief gels • Natural fragrance formulations.
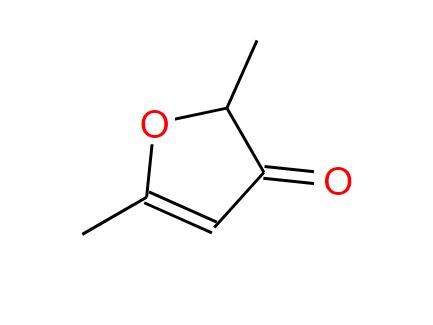
Ethyl Salicylate (CAS: 118-61-6) is an organic ester formed by the reaction of salicylic acid with ethanol. Here's a concise chemical profile:
Formula: C₉H₁₀O₃
IUPAC Name: Ethyl 2-hydroxybenzoate
Structure:
<span style="color:lightgreen;">COOCH₂CH₃</span> group attached to a benzene ring with an <span style="color:lightblue;">ortho-OH</span> group.
Appearance: Colorless to pale yellow liquid.
Odor: Sweet, wintergreen-like (similar to methyl salicylate but milder).
Solubility:
Insoluble in water.
Miscible with alcohols, oils, and organic solvents.
Found in wintergreen leaves, cloves, and some fruits.
Industrially synthesized via esterification of salicylic acid and ethanol (acid-catalyzed).
Fragrance Industry:
Base note in perfumes, soaps, and cosmetics for its sweet, balsamic scent.
Topical Analgesics:
Penetrates skin to relieve muscle/joint pain (converted to salicylic acid locally).
Flavoring Agent:
FDA-approved for foods (e.g., candies, gums) in trace amounts.
Preservative & Solvent:
Extends shelf life in cosmetics; dissolves active ingredients.
Low toxicity (vs. methyl salicylate), but concentrated forms may irritate skin/mucous membranes.
Avoid ingestion (metabolizes to salicylates – aspirin-related compounds).

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية