Search

Search











2'-Deoxy-L-thymidine is an HBV reverse transcriptase inhibitor used to treat hepatitis B infections.
2'-Deoxy-L-thymidine is a synthetic thymine deoxynucleoside drug against hepatitis B virus DNA polymerase. 2'-Deoxy-L-thymidine is phosphorylated to the active metabolite adenosine under the action of cytokinase, which inhibits the activity of hepatitis B virus DNA polymerase by competing with thymine 5′-adenosine, a natural substrate in hepatitis B virus; By integrating into the hepatitis B virus DNA, it causes the hepatitis B virus DNA strand to be prolonged and terminated. It simultaneously inhibits the synthesis of the first and second strands of hepatitis B virus DNA. It is indicated for the treatment of adult patients with chronic hepatitis B who have evidence of hepatitis B virus activity replication and are compensated for liver function with persistently elevated aminotransferases or active lesions of the liver.
Items | Specification | Result |
Assay | ≥ 99.5% | 99.91% |
Appearance | white fine powder | Conforms |
Loss on drying | ≤0.5% | 0.11% |
Welcome to inquiry us to get the complete COA!
| Product parameters | |
| Cas number: | 3424-98-4 |
| Appearance: | white fine powder |
| Purity: | 99.5%min |
| Package details: | 10g/aluminium foil bag;25kg/drum |
| Brand: | Fortunachem |
2'-Deoxy-L-thymidine is the synthetic, mirror-image (L-enantiomer) form of the natural nucleoside deoxythymidine (which is the "D-" form and a fundamental building block of DNA). Because of its reversed chirality, it is not recognized or used by human enzymes involved in DNA synthesis. This property makes it biologically inactive in most standard systems but also interesting for developing therapeutic oligonucleotides (like aptamers or Spiegelmers) that are highly stable and resistant to degradation in the body.
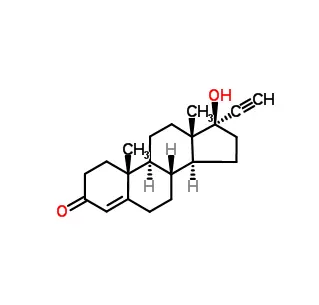
Core Structure: It consists of the nucleobase thymine attached to the sugar deoxyribose.
Key Feature (The "L"): The crucial difference from natural thymidine lies in the chirality (handedness) of the deoxyribose sugar. Natural DNA is built exclusively from D-deoxyribose. This compound is its non-natural L-enantiomer—its exact mirror image.
Relationship: It is the enantiomer of the natural DNA nucleoside, 2'-deoxy-D-thymidine (almost always just called "deoxythymidine" or "dT").
The "L" configuration completely changes its biological behavior:
Biological Inertness: The enzymes that build and read DNA (DNA polymerases, kinases, etc.) are stereospecific; they are evolved to recognize and process only the D-form sugars. L-nucleosides like this one are essentially "invisible" to these enzymes.
Resistance to Degradation: For the same reason, they are highly resistant to degradation by the body's nucleases (enzymes that break down nucleic acids). This confers great metabolic stability.
Its unique properties make it valuable for specific advanced applications:
Spiegelmers: This is its most prominent application. Spiegelmers (from German "Spiegel" for mirror) are synthetic L-oligonucleotide aptamers. Like conventional aptamers (made from D-nucleotides), they can bind to specific target molecules (e.g., proteins) with high affinity. However, because they are made of L-nucleotides, they are invisible to the human immune system and highly stable in biological fluids, making them excellent potential therapeutic drugs or diagnostic agents.
Enzyme Studies: Used as a tool in biochemistry to study the stereospecificity of enzymes that process nucleotides.
Antiviral Prodrugs: While inactive itself, certain modified L-nucleoside analogs (e.g., lamivudine) are used as prodrugs. They are activated by viral enzymes (which sometimes have different specificity) to inhibit viral replication.
| Feature | 2'-Deoxy-L-thymidine (L-dT) | 2'-Deoxy-D-thymidine (Natural dT) |
|---|---|---|
| Chirality | L-enantiomer (mirror image) | D-enantiomer |
| Role in Nature | Not found in nature | Essential building block of DNA |
| Substrate for Human Enzymes | No (not recognized) | Yes (incorporated into DNA) |
| Stability in Serum | Very High (resistant to nucleases) | Low (rapidly degraded by nucleases) |
| Primary Use | Research, Spiegelmer therapeutics | Biology, DNA synthesis, life itself |
2'-Deoxy-L-thymidine is not a natural biological molecule but a sophisticated synthetic tool. Its value lies entirely in its "unnatural" mirror-image structure, which provides metabolic stability and evasion of human enzymatic processes. This makes it a critical component in the emerging field of Spiegelmer technology, aimed at creating a new class of stable and effective oligonucleotide-based therapeutics.


Guaranteed the purity
High quality & competitive price
Quality control
Fast feedback
Prompt shipment


Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية