Search

Search











Valdecoxib is used as an intermediate . Valdecoxib is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic properties in animal models. It is a potent and selective inhibitor of prostaglandin synthesis primarily through inhibition of COX-2. Valdecoxib is used to relieve some symptoms caused by arthritis (rheumatism), such as inflammation, swelling, stiffness, and joint pain.
| Items | Specification |
| Product name | Valdecoxib |
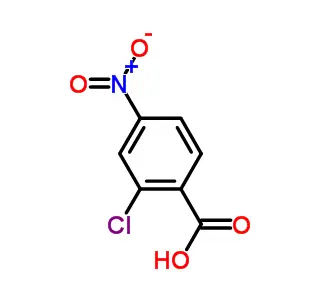
| CAS | 181695-72-7 |
| Appearance | White to off-white powder |
| Assay | ≥99% |
| Molecular Formula | C16H14N2O3S |
| Molecular Weight | 314.36 |
| EINECS | 803-082-3 |
| Product parameters | |
| Cas number: | 181695-72-7 |
| Appearance: | White to off-white powder |
| Purity: | 99%min |
| Package details: | 100g,1kg/foil bag |
| Brand: | Fortunachem |
Valdecoxib is a prescription-only nonsteroidal anti-inflammatory drug (NSAID) that belongs to the COX-2 inhibitor class (coxibs). It was marketed under the brand name Bextra® by Pfizer. It was approved for treating pain and inflammation from osteoarthritis, rheumatoid arthritis, and menstrual cramps (dysmenorrhea). However, due to serious safety concerns, it was voluntarily withdrawn from the global market in 2005.
Chemical Class: Sulfonamide-containing diaryl-substituted pyrazole. It is a selective COX-2 inhibitor.
Mechanism of Action: It works by selectively blocking the cyclooxygenase-2 (COX-2) enzyme. This enzyme is responsible for producing prostaglandins, which are chemicals that promote inflammation, pain, and fever. By inhibiting COX-2 more than COX-1, it was designed to provide anti-inflammatory benefits with a lower risk of stomach ulcers and bleeding compared to traditional NSAIDs like ibuprofen or naproxen.
Therapeutic Use: It was effective for:
Osteoarthritis
Rheumatoid Arthritis
Primary Dysmenorrhea (painful menstrual periods)
Administration: Oral tablets.
Benefit (Theoretical): Its high selectivity for COX-2 meant it offered potent anti-inflammatory and analgesic effects while minimizing the gastrointestinal toxicity (e.g., ulcers) associated with non-selective NSAIDs that also block the protective COX-1 enzyme.
Valdecoxib's withdrawal was not due to a lack of efficacy but because of an unacceptable risk of severe, life-threatening adverse events:
Serious Skin Reactions: Including fatal cases of Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN), which cause severe skin peeling and blistering.
Cardiovascular Risks: Like other COX-2 inhibitors (e.g., rofecoxib/Vioxx®), it was associated with an increased risk of heart attack (myocardial infarction), stroke, and blood clots.
Other Risks: It also carried warnings about rare but serious allergic reactions.
The U.S. Food and Drug Administration (FDA) requested its withdrawal after determining that the drug's overall risks outweighed its benefits. Pfizer complied, removing Bextra from the market in April 2005.
| Drug Name | Brand Name (Example) | COX Selectivity | Market Status | Key Risk |
|---|---|---|---|---|
| Valdecoxib | Bextra | Selective COX-2 Inhibitor | Withdrawn (2005) | Serious skin reactions, CV events |
| Rofecoxib | Vioxx | Selective COX-2 Inhibitor | Withdrawn (2004) | Cardiovascular events |
| Celecoxib | Celebrex | Selective COX-2 Inhibitor | Still on the market | CV events (has boxed warning) |
| Ibuprofen | Advil, Motrin | Non-selective (COX-1 & COX-2) | On the market | GI bleeding, kidney issues |
| Naproxen | Aleve, Naprosyn | Non-selective (COX-1 & COX-2) | On the market | GI bleeding (lower CV risk than coxibs) |
Valdecoxib is a former COX-2 inhibitor painkiller that was effective but removed from sale worldwide due to its link to rare but extremely serious skin reactions and an increased risk of heart attack and stroke. Its history is a significant case study in pharmacovigilance (drug safety monitoring), demonstrating the importance of post-market surveillance to detect rare adverse events that may not be apparent in pre-approval clinical trials.



Guaranteed purity
High quality & competitive price
Quality control
Fast feedback
Prompt shipment

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية