Search

Search











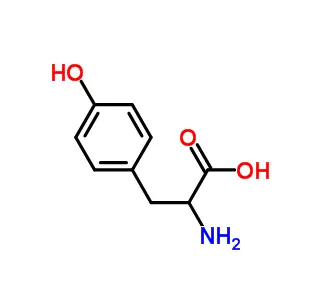
N-Cbz-O-tert-butyl-L-serine is a protected derivative of the amino acid L-serine, used as a building block in peptide synthesis. The "N-Cbz" group protects the amine (-NH₂) from reacting, while the "O-tert-butyl" group protects the reactive hydroxyl (-OH) side chain. These protections prevent unwanted side reactions, allowing chemists to selectively couple the molecule's free carboxylic acid to other amino acids. The two protecting groups can be removed under different conditions (acid or hydrogenation), providing precise control for constructing complex peptides. It is essential for synthesizing peptides containing serine.
In short, N-Cbz-O-tert-butyl-L-serine is a protected version of the natural amino acid L-Serine.
Think of it as a "Lego block" for building peptides, where two of its reactive parts have been covered with protective caps (the "Cbz" and the "tert-butyl" groups) to prevent them from reacting at the wrong time during the complex building process.
Let's dissect the name from right to left:
This is the common, natural amino acid Serine.
Its structure has three functional groups that are important for chemists:
Amino Group (-NH₂): Reactive and basic.
Carboxylic Acid Group (-COOH): Reactive and acidic.
Hydroxyl Group (-OH) on the side chain: Also reactive.
In peptide synthesis, if you try to link the amino group of one serine to the acid group of another, the side chain -OH group can also react, creating unwanted by-products. To avoid this, the reactive groups must be "protected."
N- means the protecting group is attached to the Nitrogen of the amino group.
Cbz stands for Carboxybenzyl.
This group transforms the -NH₂ into -NH-Cbz, making it less reactive.
It is a base-labile protecting group, meaning it can be removed later under mild basic conditions (like hydrogenation).
O- means the protecting group is attached to the Oxygen of the side chain's hydroxyl (-OH) group.
tert-butyl is a bulky hydrocarbon group (CH₃)₃C-.
This group transforms the -OH into a -O-tert-butyl ether, making it much less reactive.
It is an acid-labile protecting group, meaning it can be removed later with a strong acid like trifluoroacetic acid (TFA).
The primary use is in Solid-Phase Peptide Synthesis (SPPS), the standard method for making custom peptides.
Orthogonal Protection: The key feature is that the two protecting groups (Cbz and tert-butyl) are removed by completely different mechanisms (base vs. acid). This allows a chemist to remove one protecting group without affecting the other, providing precise control over the synthesis.
Step-wise Assembly: The free carboxylic acid (-COOH) can be activated to couple with the amino group of the next amino acid in the peptide chain. Meanwhile, the side chain and the other amino group are safely protected and won't interfere.
Solubility: The protecting groups often make the amino acid more soluble in organic solvents used for synthesis.
Appearance: Typically a white crystalline powder.
Protecting Group Removal:
N-Cbz Group: Removed by hydrogenolysis (H₂, Pd/C catalyst) or by treatment with strong acids like HBr in acetic acid.
O-tert-butyl Group: Removed by strong acids like Trifluoroacetic Acid (TFA) or HCl in an organic solvent.
Storage: Should be stored in a cool, dry place, protected from moisture.
N-Cbz-O-tert-butyl-L-serine is a strategically "caged" form of serine, designed for the controlled, step-by-step construction of peptides. Its two different protecting groups allow chemists to build complex molecules with high precision and yield.
It's a fundamental building block for researchers in medicinal chemistry, biochemistry, and drug discovery who are creating peptides that contain serine.




Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية