Search

Search











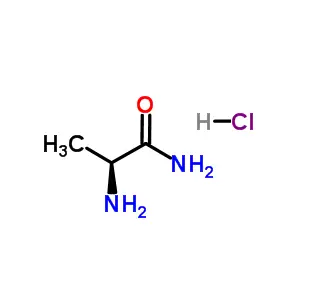
Fmoc-Leu-OH is a protected amino acid derivative used in solid-phase peptide synthesis (SPPS). It consists of the amino acid L-Leucine (Leu)—which has a hydrophobic isobutyl side chain—with its alpha-amine group protected by a 9-fluorenylmethoxycarbonyl (Fmoc) group. This temporary protecting group prevents unwanted reactions during the stepwise chemical assembly of peptides. The carboxylic acid group (-OH) remains reactive and is used to form peptide bonds with subsequent amino acids. After coupling, the Fmoc group is easily removed with a mild base like piperidine, allowing the chain to elongate. This compound is essential for synthesizing custom peptides in biomedical and pharmaceutical research.
Fmoc-Leu-OH is a protected amino acid derivative and an essential building block used in the field of solid-phase peptide synthesis (SPPS), the standard method for creating custom peptides in a laboratory. Its name is a combination of its components:
Leu: The standard three-letter abbreviation for the amino acid L-Leucine. Leucine is an essential, hydrophobic (water-repelling) amino acid with an isobutyl side chain.
Fmoc: Stands for 9-Fluorenylmethoxycarbonyl. This is a protecting group covalently attached to the alpha-amine group (-NH₂) of the leucine molecule.
-OH: Indicates that the carboxylic acid group (-COOH) of the leucine is left free and reactive.
In essence, Fmoc-Leu-OH is a leucine molecule that has been chemically modified so that its amine end is temporarily "capped" or protected, while its acid end remains ready to form a chemical bond.
The sole purpose of Fmoc-Leu-OH is to serve as a single, controlled unit for the machine-assisted, step-by-step construction of peptide chains. The process addresses a fundamental challenge in chemistry: how to connect specific amino acids in a precise order without creating a chaotic mixture.
The Problem: Amino acids have two reactive ends: an amine group (-NH₂) and a carboxylic acid group (-COOH). To form a peptide bond, the amine of one must react with the acid of another. If both ends are free, they will react indiscriminately.
The Solution with Fmoc-Leu-OH: The Fmoc group protects the amine group of leucine. This prevents it from reacting, allowing chemists to direct the reaction exclusively through its carboxylic acid group.
How it's Used in the SPPS Cycle:
Peptide synthesis is a repetitive, automated cycle:
Deprotection: The Fmoc group is removed from the amino acid currently attached to a solid resin bead using a mild base (like piperidine). This exposes its reactive amine group.
Coupling: Fmoc-Leu-OH (or another Fmoc-amino acid), along with special activating agents, is added. The exposed amine group on the resin attacks the activated carboxylic acid group of the Fmoc-Leu-OH, forming a new peptide bond and adding a leucine residue to the growing chain.
Washing: The reaction vessel is washed to remove all excess reagents and byproducts.
This cycle repeats for each amino acid in the desired sequence.
Orthogonal Protection: The Fmoc group is stable under acidic conditions but is easily and cleanly removed by a mild base. This is crucial because it allows the temporary Fmoc protection (on the amine) to be manipulated independently of other permanent protecting groups that guard the side chains of certain amino acids (e.g., on arginine or aspartic acid), which are acid-sensitive.
Hydrophobicity: Leucine's isobutyl side chain makes it highly hydrophobic. Incorporating Fmoc-Leu-OH into a peptide sequence can significantly influence the peptide's overall solubility and tendency to aggregate during synthesis, which is a key consideration for the chemist.
UV Activity: The Fmoc group absorbs ultraviolet (UV) light strongly. This property is exploited during synthesis by using UV monitors to track the completion of the deprotection step in real-time, ensuring each cycle is efficient before moving to the next coupling.
High Purity: For successful synthesis, Fmoc-Leu-OH must be of extremely high purity. Any impurities can lead to failed coupling reactions, resulting in an incorrect peptide sequence with missing amino acids (deletion sequences).
Fmoc-Leu-OH is an indispensable reagent in:
Pharmaceutical Research & Development: Synthesizing peptide-based drug candidates for a vast range of conditions, from metabolic diseases to cancer.
Academic Biochemistry: Creating specific peptide fragments to study protein-protein interactions, enzyme mechanisms, and structural biology.
Biotechnology: Developing novel peptides for diagnostic assays, vaccines, and specialized biomaterials.
In summary, Fmoc-Leu-OH is not a drug but a critical synthetic reagent. It is the protected, ready-to-use form of leucine that enables the precise, automated assembly of custom peptides, which are powerful tools in modern therapeutic and scientific research.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية