Search

Search

-Ornithine_hydrochloride主图.jpg)




-Ornithine_hydrochloride主图.jpg)




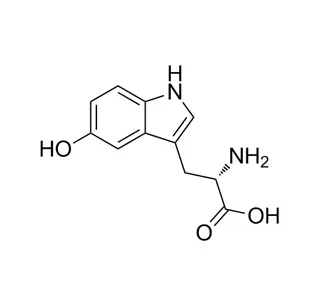
L(+)-Ornithine hydrochloride is the hydrochloride salt form of the naturally occurring L-enantiomer of the amino acid ornithine (chemical formula: C₅H₁₃ClN₂O₂).
Ornithine: A non-proteinogenic amino acid (C₅H₁₂N₂O₂), crucial in the urea cycle for detoxifying ammonia and a precursor to compounds like arginine and polyamines.
L(+): Specifies the biologically active, "left-handed" stereoisomer that is dextrorotatory (+).
Hydrochloride (HCl): The salt form improves water solubility and stability. The free base's two amino groups bind HCl, forming a crystalline powder (⁺H₃N-CH₂-CH₂-CH₂-CH(NH₃⁺)-COOH Cl⁻).
It's used clinically and in supplements due to its solubility.
L(+)-Ornithine hydrochloride is the hydrochloride salt of the naturally occurring L-enantiomer of the amino acid ornithine. Here's a detailed chemical breakdown:
Ornithine:
Chemically known as 2,5-diaminopentanoic acid.
Formula: C₅H₁₂N₂O₂
Structure: H₂N-CH₂-CH₂-CH₂-CH(NH₂)-COOH
A non-proteinogenic (not directly incorporated into proteins during synthesis) α-amino acid.
Plays a crucial role in biological systems, primarily in the urea cycle where it helps convert toxic ammonia into urea for excretion. It's also a precursor to amino acids like arginine, proline, and polyamines (e.g., putrescine, spermidine, spermine).
L(+) Enantiomer:
L: This specifies the stereochemistry (chirality) at the α-carbon atom (the carbon adjacent to the carboxyl group -COOH). The "L" designation refers to the absolute configuration similar to L-glyceraldehyde. In biological systems, amino acids are almost exclusively found in the L-form and are the physiologically active form. L-Ornithine is the natural form involved in the urea cycle.
(+): This indicates the compound is dextrorotatory, meaning it rotates the plane of plane-polarized light to the right (clockwise). While most L-amino acids are dextrorotatory (+), this is not an absolute rule (e.g., L-alanine is (+), L-serine is (+), but L-arginine is (-)). For L-ornithine, the specific rotation is positive.
Hydrochloride Salt (HCl):
Solubility: Makes them highly soluble in water, which is crucial for biological applications and formulation (e.g., in IV solutions or supplements).
Stability: Enhances crystalline stability and shelf life.
Handling: Creates a stable, typically crystalline solid.
This means the ornithine molecule is combined with hydrochloric acid (HCl).
Ornithine has two amino groups (one α-amino and one side-chain δ-amino) and one carboxyl group. In aqueous solution near physiological pH, it exists as a dication (H₃N⁺-CH₂-CH₂-CH₂-CH(NH₃⁺)-COOH), carrying two positive charges.
The hydrochloride salt is formed when the free base form of L-ornithine (which has one amino group deprotonated, typically H₂N-CH₂-CH₂-CH₂-CH(NH₃⁺)-COO⁻, making it a zwitterion with a net +1 charge) reacts with HCl. This protonates the second amino group, forming the dication, and adds a chloride anion (Cl⁻) as the counterion to achieve electrical neutrality.
Formula: C₅H₁₂N₂O₂ · HCl (Often written as C₅H₁₃ClN₂O₂ to show the protonated state)
Why a salt? Converting amino acids to their hydrochloride salts significantly improves:
Summary:
L(+)-Ornithine hydrochloride is the water-soluble hydrochloride salt of the naturally occurring, biologically active, dextrorotatory enantiomer of the amino acid ornithine. Its chemical structure is best represented as:
[⁺H₃N-CH₂-CH₂-CH₂-CH(NH₃⁺)-COOH] Cl⁻
Key Characteristics:
Chemical Name: (S)-2,5-Diaminopentanoic acid hydrochloride
Molecular Formula: C₅H₁₃ClN₂O₂
CAS Number: 3184-13-2
Appearance: Typically a white crystalline powder.
Solubility: Highly soluble in water.
Role: Important intermediate in metabolism (urea cycle, arginine/polyamine synthesis), used in research, clinical nutrition (e.g., liver support), and sometimes in dietary supplements (e.g., athletic performance). The hydrochloride form ensures good solubility for these applications.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية 
主图.jpg)