Search

Search











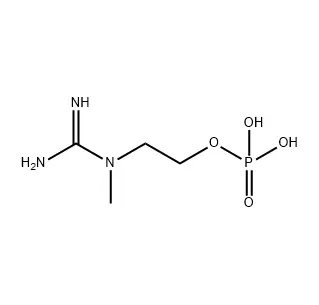
Fmoc-Pro-OH is a protected amino acid derivative used in solid-phase peptide synthesis. It consists of L-Proline (Pro)—a unique amino acid with a cyclic structure that forms a secondary amine—with its amine group blocked by a 9-fluorenylmethoxycarbonyl (Fmoc) group. This protection enables controlled, stepwise peptide chain elongation by allowing the free carboxylic acid (-OH) to form peptide bonds. The Fmoc group is later removed with a mild base. Its key role is to incorporate proline’s distinct conformational properties (e.g., inducing bends) into synthetic peptides for drug development and biochemical research.
Fmoc-Pro-OH is a protected amino acid derivative and a fundamental building block used in solid-phase peptide synthesis (SPPS), the primary method for chemically creating custom peptides in a lab. Its name is a combination of its components:
Pro: The standard three-letter abbreviation for the amino acid L-Proline. Proline is unique among the standard amino acids because its side chain forms a cyclic structure that connects back to its own alpha-amine group, making it a secondary amine.
Fmoc: Stands for 9-Fluorenylmethoxycarbonyl. This is a protecting group attached to the secondary alpha-amine group of the proline molecule.
-OH: Indicates that the carboxylic acid group (-COOH) of the proline is free and reactive.
In simple terms, Fmoc-Pro-OH is a proline molecule that has been prepared for controlled use in a chemical assembly line, with its reactive amine group temporarily masked.
The purpose of Fmoc-Pro-OH is to serve as a single, controlled unit for the stepwise construction of peptide chains. Its use is critical for incorporating proline into a specific position within a peptide sequence.
The Challenge with Proline:
Proline's unique structure (a secondary amine) gives it distinct properties. In a peptide chain, it is often a "helix breaker" and introduces kinks or turns, influencing the entire 3D structure of the peptide. From a synthesis perspective, its secondary amine is less nucleophilic (less reactive) than the primary amines of other amino acids, which can sometimes make coupling reactions slower or less efficient.
The Solution with Fmoc-Pro-OH:
The Fmoc group protects the secondary amine group of proline. While this is its main job, the preparation of Fmoc-Pro-OH also often involves "activating" the proline to make it more reactive for the upcoming coupling step.
How it's Used in the SPPS Cycle:
The process is part of an automated cycle:
Deprotection: The Fmoc group is removed from the amino acid currently attached to a solid resin bead using a mild base (like piperidine), exposing its reactive amine group.
Coupling: Fmoc-Pro-OH, along with potent activating agents, is added. The exposed amine group on the resin attacks the activated carboxylic acid group of the Fmoc-Pro-OH, forming a new peptide bond and adding a proline residue to the growing chain. Due to proline's lower reactivity, coupling may require longer reaction times or more efficient activating agents.
Washing: Excess reagents and byproducts are washed away.
This cycle repeats for each amino acid in the desired sequence.
Inducing Secondary Structure: The primary importance of using Fmoc-Pro-OH is to introduce proline's unique conformational properties into a synthetic peptide. Proline is crucial for creating beta-turns and disrupting regular alpha-helix or beta-sheet structures, which is essential for designing peptides with specific biological activities and shapes.
Orthogonal Protection: Like other Fmoc-protected amino acids, the Fmoc group on proline is stable under acidic conditions but easily removed by a mild base. This allows it to be used alongside other acid-labile protecting groups on different amino acid side chains.
Reduced Risk of Racemization: A significant advantage of using the Fmoc strategy with proline is that it has a very low tendency to undergo racemization (losing its chiral integrity and forming the D-isomer) during coupling. This is a common problem with other protection schemes and makes Fmoc-Pro-OH highly reliable for producing stereochemically pure peptides.
Coupling Considerations: Chemists are aware that coupling reactions involving proline can be slower. They often use high-performance activating agents like HATU or HBTU to ensure the reaction goes to completion efficiently.
Fmoc-Pro-OH is an indispensable reagent in:
Pharmaceutical Research: Synthesizing peptide-based drugs where proline's role in creating specific bioactive conformations is critical. This includes drugs for cardiovascular disease, cancer, and metabolic disorders.
Academic Research: Studying protein folding, stability, and structure-activity relationships by strategically placing proline residues into synthetic peptide sequences.
Biotechnology: Creating peptides for diagnostic tools, enzymes, and receptors where the structural bend induced by proline is essential for function.
In summary, Fmoc-Pro-OH is the protected, ready-to-use form of the unique amino acid L-Proline. It is a critical reagent for incorporating proline's distinct structure-shaping properties into synthetic peptides, which is vital for developing new therapeutics and understanding protein function.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية