Search

Search











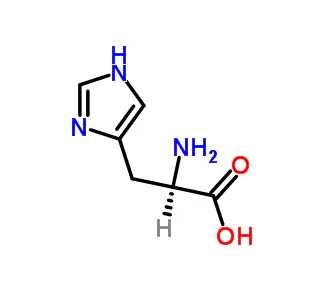
FMOC-Ala-OH is a protected amino acid derivative used in solid-phase peptide synthesis (SPPS). It consists of the amino acid Alanine (Ala) with its amine group blocked by a Fluorenylmethyloxycarbonyl (Fmoc) protecting group. This prevents unintended reactions during peptide chain assembly. The carboxylic acid group (-OH) remains active, allowing it to form peptide bonds with other amino acids. The Fmoc group is later removed using a mild base, exposing the amine for further elongation. It is a essential reagent for constructing custom peptides in biomedical and chemical research.
Items | Specifications | Results |
Appearance | Conforms | |
Purity(HPLC) | ≥99% | 99.7% |
Assay (AT) | ≥98.5% | 100.2% |
0.5mmole in 5m1 DMF clearly soluble | clearly soluble | |
Specific rotation[α]D20 | -18.0 ±2.0° | -18.1° |
Optical Purity | ≤0.30% D-enantiomer | 0.02% |
Water | ≤6.0% | 5.7% |
Any other impurity | ≤0.30% | 0.15% |
IR Spectrum | In accordance with the structure | Complies |
Acetic acid ions (IC) | ≤100mg/kg | 38.44mg/kg |
Conclusion | The product conforms to the Enterprise Standard. | |
FMOC-Ala-OH (more accurately written as Fmoc-Ala-OH) is a fundamental building block used in the synthetic chemistry field of solid-phase peptide synthesis (SPPS). It is the derivative of the amino acid L-Alanine (symbol: Ala) that has been specially modified for controlled chemical reactions. Its name is a combination of its components:
Ala: The standard three-letter abbreviation for the amino acid Alanine.
OH: Indicates the carboxylic acid functional group of alanine is present and free to react.
Fmoc: Stands for 9-Fluorenylmethoxycarbonyl. This is a protecting group attached to the amine functional group (-NH₂) of the alanine.
In essence, Fmoc-Ala-OH is an alanine molecule where its carboxylic acid end is ready for action, but its amine end is temporarily masked.
The central purpose of Fmoc-Ala-OH is to serve as a single, reliable "link" in the chain when scientists are constructing custom peptides (short chains of amino acids) and proteins in the laboratory.
The Need for Protection: To build a peptide, amino acids are connected in a specific sequence by forming a bond between the amine group of one amino acid and the carboxylic acid group of another. If both functional groups were reactive at the same time, the amino acids would react haphazardly, forming a useless mixture. The Fmoc group protects the amine group of alanine, preventing this from happening.
The Synthesis Cycle: In Fmoc-based SPPS, the C-terminus of the first amino acid is anchored to an insoluble resin bead. The synthesis then proceeds cycle-by-cycle:
Deprotection: The Fmoc group of the attached amino acid is removed with a mild base (typically piperidine), exposing its reactive amine group.
Coupling: A solution containing Fmoc-Ala-OH (or another Fmoc-protected amino acid) is added. With the help of activating agents, the now-exposed amine group on the resin attacks the reactive carboxylic acid group of Fmoc-Ala-OH, forming a new peptide bond and adding alanine to the chain.
Washing: The byproducts and excess reagents are washed away.
This cycle repeats for each additional amino acid in the desired sequence.
Orthogonal Protection: The Fmoc group is stable under acidic conditions but is easily and cleanly removed by a mild base. This allows it to be used alongside other acid-labile protecting groups for the side chains of certain amino acids (e.g., for Asp, Glu, Lys), giving chemists tremendous flexibility to synthesize complex peptides.
Crystalline Solid: Fmoc-Ala-OH is typically a white, crystalline powder. This makes it easy to handle, weigh accurately, and store stably.
UV-Active: The Fmoc group has a strong absorbance in the ultraviolet (UV) range. This property is exploited during synthesis to monitor the efficiency of the deprotection step in real-time.
Purity: High chemical purity is critical for Fmoc-Ala-OH to ensure efficient coupling and prevent the formation of deletion sequences (peptides missing an amino acid) in the final product.
Fmoc-Ala-OH is an indispensable reagent in:
Pharmaceutical Research: Synthesizing peptide-based drug candidates for treating diseases like diabetes, cancer, and obesity.
Academic Research: Creating specific peptides to study protein-protein interactions, enzyme function, and cellular signaling pathways.
Biotechnology: Developing novel peptides for diagnostic tools, vaccines, and materials science.
In summary, Fmoc-Ala-OH is not a drug or a naturally occurring compound, but a critical engineered reagent that enables the precise, machine-assisted construction of custom peptides, which are vital tools in modern drug discovery and biochemical research.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية