Search

Search










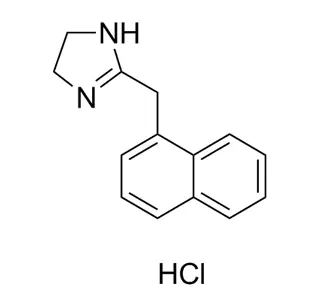
(+/-)-Narwedine is a 50/50 (racemic) mixture of two mirror-image forms of an organic compound. It is a crucial synthetic intermediate for producing galantamine, a medication for Alzheimer's disease.
Only one enantiomer, (-)-narwedine, leads to the active drug. The racemic mixture is used industrially because it can undergo dynamic kinetic resolution, a efficient process that favors the production of the desired, active enantiomer of galantamine during synthesis, making large-scale production feasible.
Items | Specifications | Results |
Appearance | off-white powder | |
Heavy metals(Pb) | ≤20ppm | <20ppm |
Loss on drying | ≤1.0% | 0.35% |
Related substance(HPLC) | Single impurities ≤1.0% Total impurities ≤2.0% | 0.58% 1.59% |
Purity(HPLC) | ≥98.0% | 98.41% |
Conclusion | The substance (+/-)-Narwedine of batch No.JLXN20240401 complies with the Requirements of In House Specifications.
| |
(+/-)-Narwedine is a racemic mixture of a key organic compound that serves as a crucial intermediate in the chemical synthesis of galantamine, a medication used to treat Alzheimer's disease. The "(+/-)" symbol indicates that it is a 50/50 mix of two mirror-image forms (enantiomers) of the molecule.
(±) or (+/-): This prefix is chemical notation for a racemic mixture. It means the sample contains equal parts of the two enantiomers of the narwedine molecule.
Enantiomers are molecules that are non-superimposable mirror images of each other, much like your left and right hands. They are labeled as the (+)-enantiomer (which rotates plane-polarized light clockwise) and the (-)-enantiomer (which rotates it counterclockwise).
Narwedine: This is the name of the specific chemical compound. It has a defined molecular structure based on a fused ring system (a benzofuranone coupled with a cyclohexene ring).
So, (+/-)-Narwedine is a 1:1 mixture of (+)-Narwedine and (-)-Narwedine.
Narwedine's primary importance lies in its relationship to the natural product galantamine (also known as galanthamine).
Galantamine is a biologically active compound originally isolated from certain daffodils and snowdrop bulbs. It is an acetylcholinesterase inhibitor, meaning it increases levels of the neurotransmitter acetylcholine in the brain, which is beneficial for treating the symptoms of mild to moderate Alzheimer's disease.
Narwedine is structurally very similar to galantamine. It is essentially a precursor or synthetic intermediate. Through a specific chemical reaction (often a reduction), narwedine can be converted into galantamine.
The critical point is that only one of the two enantiomers in the racemic mixture is useful:
(-)-Narwedine is converted into (+)-galantamine, which is the naturally occurring, biologically active form of the drug.
(+)-Narwedine is converted into (-)-galantamine, which is biologically inactive or much less active.
This relationship is why the synthesis and resolution of narwedine are so important.
The synthesis of (+/-)-Narwedine and its subsequent conversion to galantamine was a landmark achievement in organic chemistry. The most famous synthesis was published by the Polish chemist Witold Jedrzejewski (also spelled Jędrzejewski) in 1972.
The key step in his synthesis produced an intermediate now famously known as the "Narwedine Enone" or "Jedrzejewski's Enone." This molecule is the immediate precursor to (+/-)-Narwedine.
The brilliance of this route is that the racemic Narwedine Enone can undergo a process called dynamic kinetic resolution during its conversion to galantamine. This is a sophisticated technique that, under the right conditions, can favor the production of the desired active enantiomer, (+)-galantamine, from the racemic starting material, making the synthesis much more efficient.
Key to Industrial Synthesis: Because extracting galantamine from plants is inefficient and low-yielding, a reliable chemical synthesis is necessary to produce the drug on a commercial scale. The synthetic route through (+/-)-Narwedine provides a practical and scalable method.
Illustrates Important Chemical Concepts: It is a classic example used to teach advanced organic chemistry concepts, including:
Racemic Mixtures and Enantiomers
Asymmetric Synthesis
Dynamic Kinetic Resolution - a powerful method for obtaining a single enantiomer from a racemic mixture.
Historical Milestone: It represents a significant accomplishment in total synthesis, enabling the widespread availability of an important medicine.
| Feature | Description |
|---|---|
| Identity | A racemic mixture of the Narwedine molecule. |
| Significance | A crucial synthetic intermediate for the production of the Alzheimer's drug, galantamine. |
| Key Concept | Demonstrates the importance of stereochemistry in drug development: only one enantiomer (from (-)-Narwedine) leads to the active drug. |
| Context | Central to a famous industrial synthesis that relies on dynamic kinetic resolution to efficiently produce the desired single enantiomer of the final drug. |



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية