Search

Search











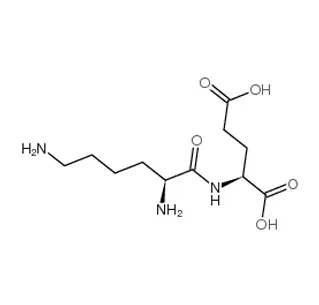
D-tert-Butylglycine (D-tBuGly) is a non-proteinogenic, synthetic amino acid used to engineer advanced peptides and drugs.
Structure: Glycine with a bulky tert-butyl group (-C(CH₃)₃) at the α-carbon in the D-configuration.
Properties:
Steric bulk forces peptides into stable folds (e.g., β-turns).
High hydrophobicity boosts cell membrane penetration.
D-form resists enzyme degradation → extends drug half-life.
Applications:
Design of antimicrobial/anticancer peptides.
Enzyme inhibitors (blocks active sites via steric clash).
Enhancing metabolic stability in peptide therapeutics.
D-tert-Butylglycine (D-tBuGly or D-Tle) is a synthetic, non-proteinogenic amino acid characterized by its specific structure and properties. Here's a breakdown:
Core Structure: It's an alpha-amino acid, meaning it has an amino group (-NH₂), a carboxylic acid group (-COOH), and a side chain attached to the central alpha-carbon.
Side Chain: Its defining feature is a bulky tert-butyl group (-C(CH₃)₃) attached directly to the alpha-carbon. This group is:
Highly hydrophobic: Repels water.
Sterically bulky: Takes up a lot of space.
Stereochemistry: The "D-" prefix specifies its stereochemistry. It is the dextrorotatory enantiomer, meaning it rotates plane-polarized light to the right. This is the mirror-image form not commonly found in natural proteins (which primarily use L-amino acids).
Chemical Structure:
O
║
HO-C-C*-NH₂
|
(CH₃)₃C
Key Properties and Significance:
High Steric Hindrance: The large, branched tert-butyl group creates significant steric bulk around the alpha-carbon. This severely restricts the conformational flexibility (rotation around bonds) of any peptide chain it's incorporated into.
Hydrophobicity: The tert-butyl group is strongly hydrophobic, making D-tBuGly and peptides containing it less soluble in water.
Unnatural Configuration: The D-configuration makes it unrecognizable to most natural biological systems that process L-amino acids (like ribosomes and many proteases).
Primary Uses in Research & Chemistry:
Peptide Engineering & Conformational Control:
Inducing/Stabilizing Beta-Turns: Its bulk and restricted flexibility are often used to force or stabilize specific turns (especially Type II' beta-turns) in peptide backbones, crucial for mimicking bioactive peptide structures.
Creating Peptidomimetics: Used to design modified peptides (peptidomimetics) with enhanced stability, altered activity, or specific 3D shapes that natural amino acids can't achieve.
Reducing Conformational Flexibility: Limits the number of possible conformations a peptide can adopt, simplifying structural studies or locking peptides into a bioactive shape.
Enhancing Proteolytic Stability: The combination of D-configuration and bulky side chain makes peptides containing D-tBuGly highly resistant to degradation by most proteolytic enzymes (proteases), increasing their half-life in vivo.
Studying Enzyme Specificity & Protein Folding: Used as a probe to understand how enzymes recognize substrates or how proteins fold, due to its unnatural size and stereochemistry.
Modulating Peptide Properties: Its hydrophobicity can be exploited to influence peptide solubility, membrane permeability, and interactions with hydrophobic targets or environments.
Synthesis:
D-tert-Butylglycine is not found naturally and is synthesized chemically in the laboratory. Common methods include:
Strecker Synthesis: Using tert-butyl aldehyde (pivalaldehyde), ammonia, and cyanide.
Alkylation of Glycine Equivalents: Alkylating a glycine synthon (like a Schiff base or oxazinone) with tert-butyl halide.
Resolution: Synthesizing the racemic mixture (DL-tert-butylglycine) and then separating the D-enantiomer using chiral techniques.
In summary: D-tert-Butylglycine is a valuable synthetic tool in peptide chemistry and drug design. Its unique combination of a bulky, hydrophobic tert-butyl group and the unnatural D-configuration provides powerful ways to control peptide conformation, enhance stability against enzymatic breakdown, and create novel bioactive molecules with tailored properties.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية