Search

Search

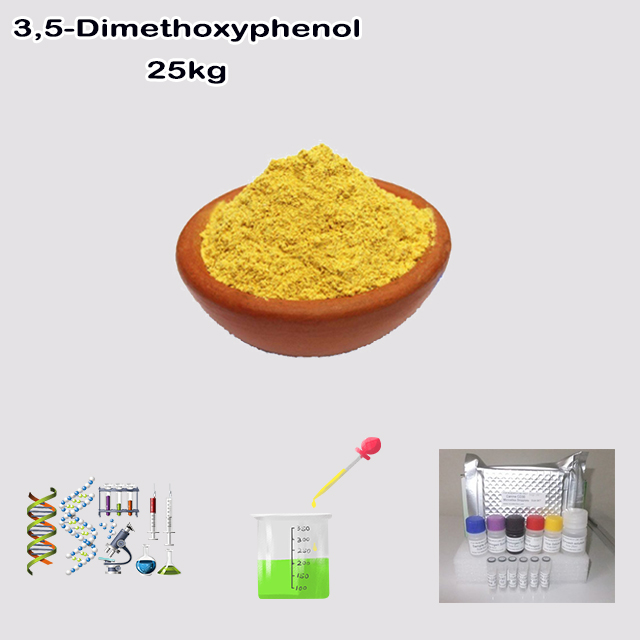









3,5-Dimethoxyphenol is an organic compound where a phenol ring has two methoxy (-OCH₃) groups at the 3 and 5 positions. This structure makes it an electron-rich building block (synthon) widely used in organic synthesis. It's a key intermediate for creating pharmaceuticals, agrochemicals, and fragrance ingredients. Typically a white crystalline solid, it requires careful handling as it is harmful and can cause irritation.
| Item | Specifications | Results |
| Appearance | Off white to orange red crystal | Orange yellow crystal |
| Assay | ≥98.0% | 98.2% |
| Product parameters | |
| Cas number: | 500-99-2 |
| Appearance: | Orange yellow crystal |
| Purity: | 98.0%min |
| Package details: | 100g/bag,1kg/bag |
| Brand: | Fortunachem |
3,5-Dimethoxyphenol is an organic compound belonging to the class of phenols. Its structure consists of a phenol ring (a benzene ring with a hydroxyl group -OH) where two methoxy groups (-OCH₃) are attached at the meta positions (carbons 3 and 5) relative to the hydroxyl group.
It is a versatile chemical building block, primarily used in organic synthesis and industrial applications.
Chemical Formula: C₈H₁₀O₃
CAS Number: 500-99-2
Molar Mass: 154.16 g/mol
Appearance: It typically appears as white to off-white crystals or a crystalline powder.
Melting Point: Approximately 78 - 81 °C (172 - 178 °F)
Solubility: It is soluble in common organic solvents like ethanol, methanol, ether, and acetone. It has limited solubility in water.
The placement of the substituents is key to its reactivity:
The hydroxyl (-OH) group makes it acidic and a potential site for reactions like deprotonation or formation of ethers and esters.
The two methoxy (-OCH₃) groups in the meta positions are strong activating and ortho/para-directing groups. This makes the aromatic ring highly electron-rich and reactive towards electrophilic aromatic substitution. The positions ortho to the methoxy groups (carbons 2, 4, and 6) are particularly susceptible to attack by electrophiles.
This high reactivity is precisely why it's such a valuable synthon.
Organic Synthesis (Most Important Use):
Formylation (e.g., using the Riemer-Tiemann reaction to create aldehydes).
Halogenation (e.g., bromination).
Coupling Reactions.
Pharmaceuticals: It is used in the research and development of various drugs.
Agrochemicals: It serves as an intermediate in the synthesis of certain herbicides, fungicides, and pesticides.
Ligands and Catalysts: It can be modified to create ligands for metal complexes used in catalysis.
Building Block for Complex Molecules: It is a crucial starting material or intermediate in the synthesis of more complex organic compounds, including:
Electrophilic Aromatic Substitution: Its electron-rich nature makes it ideal for reactions like:
Fragrance and Flavor Industry:
It is used in the synthesis of certain aroma compounds. Its derivatives can contribute woody, smoky, or spicy notes to perfumes and flavorings.
Research Chemical:
It is a common reagent in chemical research laboratories for exploring new synthetic pathways and creating novel chemical entities.
Like many phenolic compounds, 3,5-Dimethoxyphenol requires careful handling.
Hazards: It is harmful if swallowed, in contact with skin, or if inhaled. It can cause skin and eye irritation.
Precautions:
Always wear appropriate Personal Protective Equipment (PPE), including safety glasses, gloves, and a lab coat.
Work in a well-ventilated area, preferably a fume hood.
Avoid creating dust.
Storage: It should be stored in a cool, dry, well-ventilated place in a tightly closed container.
Important Note: Always consult the Safety Data Sheet (SDS) for the most accurate and detailed safety information before handling this or any chemical.
| Aspect | Description |
|---|---|
| Identity | An organic compound, specifically a disubstituted phenol. |
| Key Feature | Electron-rich aromatic ring due to two meta-oriented methoxy groups. |
| Primary Use | Versatile building block (synthon) in organic synthesis for pharmaceuticals, agrochemicals, and fragrances. |
| Appearance | White to off-white crystalline solid. |
| Handling | Requires caution; harmful and irritating. Use PPE and a fume hood. |
In essence, 3,5-Dimethoxyphenol is not a consumer product but a fundamental intermediate chemical valued for its unique reactivity, which allows chemists to construct a wide array of more complex and useful molecules.




Guaranteed the purity
High quality & competitive price
Quality control
Fast feedback
Prompt shipment

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية