Search

Search

cyclohexanecarboxylate主图.jpg)




cyclohexanecarboxylate主图.jpg)




Here's a concise explanation of N-Succinimidyl 4-(maleimidomethyl)cyclohexanecarboxylate (SMCC) within 100 words:
SMCC is a heterobifunctional crosslinker used to covalently join two different biomolecules. It features two key reactive groups:
NHS Ester: Reacts specifically with primary amines (-NH₂, e.g., on proteins' lysines) forming stable amide bonds.
Maleimide: Reacts specifically with thiols (-SH, e.g., on cysteines) forming stable thioether bonds.
The rigid cyclohexane spacer between these groups enhances the maleimide's stability against hydrolysis. SMCC enables stepwise conjugation: typically link an amine-containing molecule (like an antibody) first, then attach a thiol-containing molecule (like a drug or enzyme). It's crucial for creating Antibody-Drug Conjugates (ADCs), diagnostics, and other bioconjugates. Handle as moisture-sensitive solid.
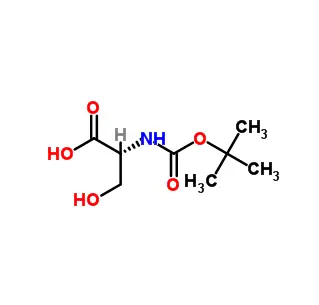
N-Succinimidyl 4-(maleimidomethyl)cyclohexanecarboxylate (SMCC) is a heterobifunctional crosslinking reagent commonly used to conjugate biomolecules. Its key features are:
Two Reactive Groups:
N-Hydroxysuccinimide (NHS) Ester: Reacts specifically with primary amines (-NH₂, e.g., on lysine residues or protein N-termini) to form stable amide bonds.
Maleimide Group: Reacts specifically with sulfhydryl groups (-SH, e.g., on cysteine residues) via thioether bonds.
Spacer: The cyclohexane ring provides a rigid, hydrophobic spacer (~11.6 Å) separating the two reactive ends. This structure enhances the stability of the maleimide group against hydrolysis compared to similar linkers with linear alkyl spacers.
Primary Function: To covalently link an amine-containing molecule (e.g., an antibody, protein, or amine-modified nucleic acid) to a thiol-containing molecule (e.g., a peptide, toxin, drug, enzyme, or thiol-modified oligonucleotide). The reaction is typically performed sequentially: NHS-ester first with the amine, then maleimide with the thiol.
Key Applications:
Antibody-Drug Conjugates (ADCs): Linking cytotoxic drugs to antibodies.
Enzyme-Antibody Conjugates: Creating immunoenzymes for ELISA/diagnostics.
Protein-Protein/Protein-Peptide Conjugates.
Immobilization: Attaching proteins/peptides to surfaces or carriers functionalized with amines or thiols.
Bioconjugation research.
Handling: Typically supplied as a solid. Soluble in anhydrous DMSO or DMF. Moisture-sensitive; store desiccated at ≤ -20°C.
In short: SMCC is a versatile, stable crosslinker designed for specific, stepwise conjugation of amine- and thiol-containing molecules, widely used in bioconjugation, especially for ADCs and diagnostics.



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية 

主图.jpg)