Search

Search











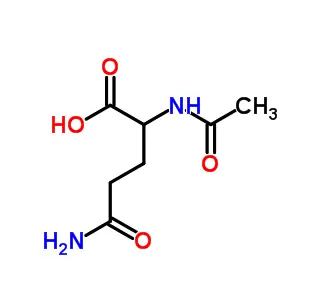
DL-2-Aminobutyric acid (DL-α-Aminobutyric acid, DL-2-AB) is a synthetic, non-proteinogenic amino acid existing as a racemic mixture (equal D- and L- forms). Its chemical structure is CH₃-CH₂-CH(NH₂)-COOH, featuring an ethyl side chain. While not used in proteins, it's significant for:
Structural similarity to GABA: Acts as a weak substrate/inhibitor for GABA-metabolizing enzymes and may interact with GABA receptors.
Metabolic role: The L-form is a biosynthetic intermediate, notably for the antibiotic Cephalosporin C.
Chiral building block: Its separated enantiomers are valuable in chemical synthesis.
Primarily used as a research tool in neuroscience and biochemistry.
DL-2-Aminobutyric acid (DL-α-Aminobutyric acid, DL-2-AB, DL-α-ABA) is a synthetic, non-proteinogenic amino acid. Here's a breakdown of its key aspects:
Chemical Structure:
It's a four-carbon, straight-chain amino acid.
The "2-" or "α-" indicates the amino group (-NH₂) is attached to the second carbon (the alpha-carbon) adjacent to the carboxylic acid group (-COOH).
Its side chain is an ethyl group (-CH₂-CH₃), making it one carbon longer than alanine (which has a methyl group -CH₃) and one carbon shorter than norvaline.
Molecular Formula: C₄H₉NO₂
Chemical Structure: CH₃-CH₂-CH(NH₂)-COOH
The "DL" Prefix:
This signifies that the compound is a racemic mixture. It contains equal amounts of both the D- and L- enantiomers (mirror-image isomers).
The L-enantiomer (L-2-Aminobutyric acid) is the naturally occurring form found in some contexts, while the D-enantiomer is less common.
The racemic DL-form is the most commonly synthesized and used form in research and industry.
Properties:
Physical State: Typically a white crystalline powder.
Solubility: Generally soluble in water.
Melting Point: Around 290-295°C (with decomposition).
Zwitterionic: Like other amino acids, it exists as a zwitterion (internal salt) in solution near neutral pH.
Key Characteristics and Uses:
Amino acid transport mechanisms.
GABA metabolism and neurotransmission.
Enzyme specificity (e.g., for transaminases or decarboxylases).
GABA Transaminase (GABA-T) Substrate/Inhibitor: It is a weak substrate for the enzyme GABA transaminase, which metabolizes GABA. It can also act as a weak inhibitor of this enzyme.
GABA Receptor Interactions: Studies suggest it may have weak agonist or modulatory effects on certain GABA receptor subtypes, though much weaker than GABA itself.
Non-Proteinogenic: It is not one of the 20 standard amino acids incorporated into proteins by ribosomes during translation.
Neurotransmitter Analogue: It is structurally similar to the inhibitory neurotransmitter GABA (γ-Aminobutyric acid). While not identical (GABA has the amino group on the gamma-carbon), DL-2-AB can interact with some GABA-related systems:
Metabolic Intermediate: The L-enantiomer is an intermediate in the biosynthesis of O-Acetylhomoserine in some microorganisms, which is a precursor to methionine and other compounds.
Precursor to Antibiotics: L-2-Aminobutyric acid is a biosynthetic precursor to certain antibiotics, such as Cephalosporin C.
Chiral Building Block: Both the D- and L-enantiomers, often separated from the racemate, are valuable as chiral synthons (building blocks) in organic synthesis and pharmaceutical manufacturing.
Research Tool: Used in biochemical and neurological research to study:
Safety:
Generally considered low toxicity for research use, but standard laboratory precautions should always be followed (avoid inhalation, ingestion, skin/eye contact).
NFPA Health Rating: 1 (Slight Hazard)
Decomposes on heating, producing toxic fumes (nitrogen oxides).
In summary: DL-2-Aminobutyric acid is a synthetic racemic mixture of a non-standard amino acid. Its primary significance lies in its structural similarity to GABA (leading to its use in neuroscience research), its role as a metabolic intermediate and antibiotic precursor (specifically the L-enantiomer), and its utility as a chiral building block in chemical synthesis.



Guaranteed purity
High quality & competitive price
Quality control
Fast feedback
Prompt shipment

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية