
Search

Search











Calcium disodium edetate dihydrate is used as a chelating agent; preservative and antioxidant.
Calcium disodium edetate dihydrate may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by liquid chromatography technique.
| Items | Specifications | Results |
| Appearance | White powder | Conforms |
| Assay | ≥98.0% | 98.38% |
| Product parameters | |
| Cas number: | 23411-34-9 |
| Appearance: | White powder |
| Purity: | 98%min |
| Package details: | 25kg/bag |
| Brand: | Fortunachem |
Calcium Disodium Edetate Dihydrate is the specific, hydrated form of the compound Calcium Disodium EDTA. It is a white, crystalline, odorless powder that is highly soluble in water.
In simple terms, it is a chelating agent—a molecule that can securely bind to metal ions (like lead, iron, or copper) through multiple contact points, forming a stable, inert complex that can then be safely excreted from the body.
The "Dihydrate" part of the name indicates that each molecule of the compound is associated with two molecules of water (H₂O). This is a common form for its stability and handling in pharmaceutical and industrial applications.
Edetate: Refers to EDTA (Ethylenediaminetetraacetic acid), the parent molecule from which it is derived.
Disodium: Indicates that two sodium (Na+) ions are part of the salt.
Calcium: Indicates that a calcium (Ca²+) ion is chelated (bound) within the center of the molecule. This is a critical feature.
Dihydrate: Means it has two water molecules associated with its crystal structure.
Imagine the EDTA molecule as a very efficient "claw" or "octopus" that grabs onto metal ions.
The "Claw": The EDTA molecule has six "grasping points" (two nitrogen atoms and four oxygen atoms) that can coordinate with a metal ion.
Swapping the Calcium: The pre-bound calcium ion in "Calcium Disodium EDTA" is held relatively weakly. When this compound encounters a heavier, more toxic metal ion with a higher affinity for the "claw" (like lead or mercury), a swap occurs.
Formation of a Complex: The toxic metal ion displaces the calcium ion and becomes tightly bound in the center of the EDTA claw. The calcium is released, and the new, stable, water-soluble complex (e.g., Lead-EDTA) is formed.
Excretion: This neutral complex is then filtered out by the kidneys and excreted in the urine, effectively removing the toxic metal from the body.
The pre-bound calcium is crucial because it prevents the chelator from accidentally depleting the body's own essential calcium stores while it seeks out the more toxic metals.
Heavy Metal Poisoning Treatment: This is its primary and most important medical application. It is used as an antidote for acute and chronic poisoning, particularly from:
Lead (Plumbism): It is a first-line treatment for severe lead poisoning, especially when lead levels are very high or symptoms are present.
Other Metals: It can also be used for poisoning from chromium, manganese, copper, and zinc, though other agents may be preferred.
Administration: It is administered slowly via intravenous (IV) infusion in a clinical setting. It is never used for self-treatment due to the risk of serious side effects, including kidney damage.
Preservative and Stabilizer: Used as a food additive (E-number E385).
Function:
Prevents Oxidation: It sequesters trace metal ions (like iron and copper) that act as catalysts in the oxidation of fats and oils, which causes rancidity and discoloration.
Preserves Color and Flavor: By preventing oxidation, it helps maintain the intended color, flavor, and texture of processed foods.
Common Foods Found In: Canned mayonnaise, salad dressings, sandwich spreads, canned beans and pulses, processed fruits and vegetables, and soft drinks.
Stabilizer: Used to improve the shelf life and stability of products by sequestering metal ions that could otherwise degrade colors, fragrances, or active ingredients.
Preservative Booster: It can enhance the effectiveness of other preservatives in the formula.
Water Softener: In textiles and cleaning products, it binds to calcium and magnesium ions in hard water.
Laboratory Reagent: Used in molecular biology to inactivate metal-dependent enzymes and as a component of buffers to control metal ion concentrations.
Medical Use: It is considered safe and effective when administered correctly by medical professionals for heavy metal poisoning. However, it is nephrotoxic (toxic to the kidneys) if infused too quickly or inappropriately. Side effects can include pain at the infusion site, fever, headache, and fatigue.
Food Use: Approved as a food additive in limited quantities. It is generally recognized as safe (GRAS) by the FDA when used within established limits. The amounts consumed in food are minuscule compared to medical doses and are considered harmless for the vast majority of people.
| Aspect | Description |
|---|---|
| What it is | A hydrated salt of EDTA, acting as a chelating agent. |
| Primary Function | Binds to and inactivates metal ions. |
| Key Medical Use | Treatment for severe heavy metal poisoning (especially lead). |
| Key Food Use | Preservative and stabilizer (E385) to prevent spoilage. |
| Safety | Medical: Potent drug with risks, requires clinical supervision. Food: Safe at regulated low concentrations. |
In essence, Calcium Disodium Edetate Dihydrate is a versatile compound whose life-saving ability to grab toxic metals is harnessed in medicine, while its milder metal-binding property is used to preserve the quality and safety of food and consumer products.

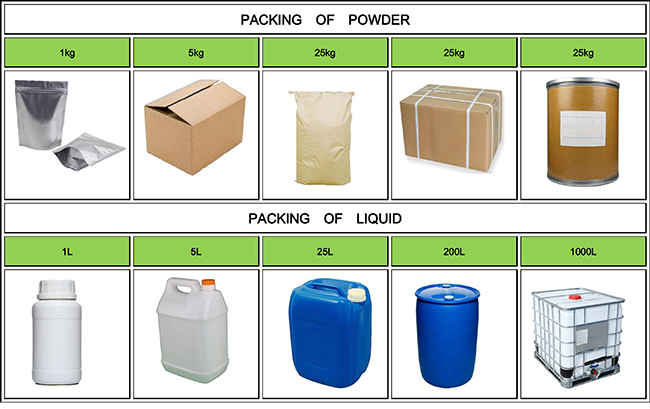


Used as a chelating agent, preservative, antioxidant, etc.

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية 






