Search

Search











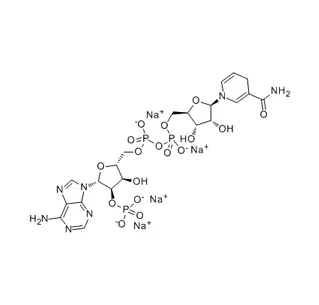
β-Nicotinamide Adenine Dinucleotide Disodium Salt is the stable, water-soluble sodium salt form of NAD⁺, a fundamental coenzyme found in all living cells. It is essential for energy metabolism, acting as a primary electron carrier in processes like glycolysis and the Krebs cycle to generate ATP. This salt form is crucial for biochemical research, diagnostic enzyme assays, and cell culture applications, where it serves as a key cofactor for dehydrogenases. Its stability makes it the preferred form for laboratory use over the pure acid.
Items | Specifications | Results |
Appearance | Complies | |
Purity | ≥98.0% | 98.3% |
Assay(Dry Basis) | ≥95.0% | 96.5% |
Sodium content | 5.5%-7.5% | 5.8% |
Water content | ≤8% | 5.9% |
PH(1.0g/100ml(25℃)) | 7.0-10.0 | 8.8 |
Heavy Metals | ||
Pb(ppm) | NMT 1ppm | Conform |
As(ppm) | NMT 1ppm | Conform |
Cd(ppm) | Conform | |
Hg(ppm) | NMT 1ppm | |
Total Plate Count | ||
Yeast&Mold | NMT 100 CFU/g | Conform |
E.coil | Negative | Negative |
Salmonella | Negative | Negative |
Conclusion | This product complies with the specifications. | |
| Product parameters | |
| Cas number: | 606-68-8 |
| Appearance: | White powder |
| Purity: | 95%; 98% |
| Package details: | 100g/bottle; 1kg/foil bag |
| Brand: | Fortunachem |
β-Nicotinamide Adenine Dinucleotide Disodium Salt is the stable, sodium salt form of NAD⁺ (Nicotinamide Adenine Dinucleotide), one of the most crucial molecules in all living cells. It is commonly abbreviated as NAD⁺ disodium salt or β-NAD⁺ Na₂.
To understand this compound, it's best to break down its name:
β-Nicotinamide Adenine Dinucleotide (β-NAD⁺): This is the full name for the coenzyme NAD⁺. It consists of two nucleotides (adenine and nicotinamide) joined together. The "β" denotes the specific three-dimensional configuration of the chemical bond connecting the nicotinamide moiety to the sugar molecule; this is the biologically active form. NAD⁺ is a coenzyme, meaning it acts as an essential helper for enzymes that catalyze vital reactions.
Disodium Salt: This indicates that the molecule has been stabilized by binding two sodium ions (Na⁺) to its phosphate groups. This salt form is more stable, less hygroscopic (absorbs less moisture), and more soluble in water than the free acid form, making it the preferred standard for laboratory and commercial use.
The fundamental role of the NAD⁺ molecule is to act as a shuttle for electrons and energy in metabolic processes. It constantly cycles between two forms:
NAD⁺ (Oxidized Form): The form provided by this disodium salt. It acts as an electron acceptor.
NADH (Reduced Form): After accepting two electrons and one proton (a hydride ion, H⁻), NAD⁺ is reduced to NADH. NADH carries this energy to another part of the cell.
Its primary biological roles are:
Energy Metabolism: This is its most famous function. NAD⁺ is a key player in glycolysis and the Krebs (Citric Acid) Cycle, where it accepts electrons during the breakdown of glucose and fats. The resulting NADH then donates these electrons to the electron transport chain to produce the bulk of the cell's ATP (adenosine triphosphate), the primary energy currency.
Cellular Repair and Signaling: NAD⁺ is a required substrate for several important enzymes, including:
Sirtuins (SIRTs): Proteins involved in DNA repair, reducing inflammation, and regulating lifespan.
PARPs (Poly-ADP-ribose-polymerases): Enzymes critical for repairing damaged DNA.
Because these enzymes consume NAD⁺, its availability directly influences cellular health, aging, and stress resistance.
The β-NAD⁺ disodium salt is not typically used as a direct dietary supplement due to poor absorption. Its main applications are scientific and industrial:
Enzyme Assays: It is an essential reagent in clinical diagnostics and biochemical research to measure the activity of hundreds of dehydrogenase enzymes (e.g., lactate dehydrogenase - LDH, alcohol dehydrogenase - ADH). The reaction rate is tracked by measuring the conversion of NAD⁺ to NADH, which absorbs light at 340 nm.
Cell Culture: It is added to cell culture media to support the metabolic needs of growing cells in the lab.
Cofactor Supplementation: Used in in vitro enzymatic synthesis to drive reactions that require NAD⁺ as a cofactor.
Research: Scientists use it to study cellular metabolism, aging, neurodegeneration, and the mechanisms of the enzymes that depend on it (sirtuins, PARPs).
In essence, β-Nicotinamide Adenine Dinucleotide Disodium Salt is the stable, commercially available form of the vital coenzyme NAD⁺. It serves as an indispensable tool in biochemistry and medicine to study and facilitate the very reactions that power life, from energy production to DNA repair. Its importance lies in its role as the oxidized, electron-accepting form of the NAD⁺/NADH redox couple.



1. Provide more energy to the cells;
2. Improve the body's metabolism;
3. Relieve the fatigue of the human body for long-term work.

Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية