Search

Search











Alpha-iso-Methylionone (CAS 127-51-5) is a synthetic fragrance compound derived from ionone, known for its woody, floral-violet scent. Key facts:
Scent Profile:
Rich woody-violet aroma with fruity undertones, used to enhance floral or oriental perfumes.
Applications:
Perfumery: Base note in fine fragrances, soaps, detergents, and cosmetics.
Fixative: Extends scent longevity by slowing evaporation.
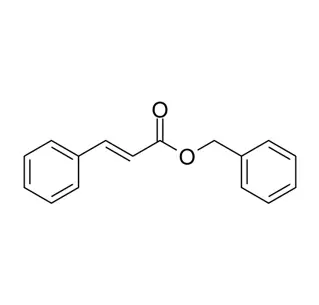
Chemical Structure:
Isomer of methylionone; differs in methyl group position on the ionone ring.
Allergen: Recognized as a skin sensitizer (EU requires labeling if ≥0.001% in leave-on products).
IFRA Compliance: Restricted to safe levels in formulations (typically ≤0.4%).
Stability: Non-photosensitizing; suitable for sun-exposed products.
Why Used: Affordable alternative to natural violet extracts, adds depth to scent compositions.
Caution: Patch-test in skincare due to sensitization risk.
alpha-iso-Methylionone (CAS 127-51-5) is a synthetic terpenoid ketone used primarily in perfumery. Here are its key chemical characteristics:
IUPAC Name:
(E)-1-(2,6,6-Trimethylcyclohex-2-en-1-yl)pent-1-en-3-one
Molecular Formula: C₁₄H₂₂O
Core Structure:
Cyclic moiety: 2,6,6-Trimethylcyclohex-2-enyl ring (derived from ionone backbone).
Chain moiety: Unsaturated ketone (α,β-unsaturated enone: −CH=CH−C(O)CH₃).
"iso" designation: Methyl group (−CH₃) attached at the C1 position of the cyclohexenyl ring (distinguishing it from α-methylionone).
Stereoisomers: Exists as cis/trans isomers due to the double bond in the side chain (the E-isomer is most common).
Chiral Centers: The cyclohexenyl ring has chiral centers at C1 and C6, leading to enantiomers (typically used as a racemic mixture).
Produced via acid-catalyzed aldol condensation:
Citral (or pseudoionone) + acetone → ionone intermediate.
Methylation: Selective allylic methylation at C1 of the ionone ring.
Functional Groups: α,β-Unsaturated enone (electrophilic, Michael acceptor).
Reactivity:
Prone to oxidation under strong conditions.
Can undergo Diels-Alder reactions (diene character in ring).
Solubility: Insoluble in water; soluble in ethanol, oils.
Boiling Point: ~295°C (decomposes).
The "iso" prefix indicates methyl substitution on the ring carbon adjacent to the side chain (C1), unlike α-methylionone where methylation is on the side chain.
IR: Strong C=O stretch at ~1670 cm⁻¹; C=C at ~1600 cm⁻¹.
NMR: Characteristic vinyl proton signals (δ 5.5–6.5 ppm).
Practical Note: Its α,β-unsaturated ketone structure contributes to both its violet-like scent and potential skin sensitization (ability to form protein adducts). This reactivity is regulated in cosmetics (IFRA limits).



Fortunachem Provides Not Only Professional Chemical Products But Also Professional Help
Keeping you up-to-date with all the latest information, news, and events about Fortunachem!

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية